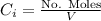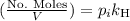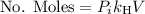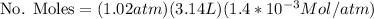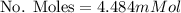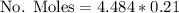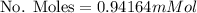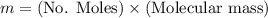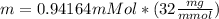Physics, 28.01.2020 20:48 Rodrigo6379
Calculate the mass of oxygen (in mg) dissolved in a 3.14 l bucket of water exposed to a pressure of 1.02 atm of air. assume the mole fraction of oxygen in air to be 0.21 and the henry's law constant for oxygen in water at this temperature to be 1.4 × 10-3 m/atm o2. (enter your value using three significant figures.)

Answers: 3


Another question on Physics

Physics, 21.06.2019 14:30
Foreshadowing. the prologue states that this is the story of "star-crossed lovers." there are four strong foreshadowings of evil in act 1. identify at least two foreshadowings and explain their purpose.
Answers: 1

Physics, 22.06.2019 06:30
Alead ball is dropped into a lake from a diving board 5m above the water . it hits the water with a certain velocity and then sink with constant velocity in bottom . it reaches the bottom 5 second after it is dropped if g= 10m/s2 . find depth of the lake and average velocity of the ball ?
Answers: 1


Physics, 22.06.2019 14:10
Click the game tab at the bottom of the simulation and select level 1. (there is no seesaw balance for this part of the activity.) balance the first equation, and click check to see if you got it right. if you can’t balance it in the first try, you can try again. work through the five equations for level 1. click continue to go on to level 2, and later level 3. each level is more difficult than the one before. keep trying until all the equations are balanced. in one or two sentences, describe how you did in the balancing game. in a few more sentences, explain one strategy you learned for balancing more complex equations.
Answers: 2
You know the right answer?
Calculate the mass of oxygen (in mg) dissolved in a 3.14 l bucket of water exposed to a pressure of...
Questions





Engineering, 12.10.2020 19:01

Chemistry, 12.10.2020 19:01

Mathematics, 12.10.2020 19:01

Mathematics, 12.10.2020 19:01

Physics, 12.10.2020 19:01



History, 12.10.2020 19:01

Mathematics, 12.10.2020 19:01



Physics, 12.10.2020 19:01

Physics, 12.10.2020 19:01

English, 12.10.2020 19:01

Biology, 12.10.2020 19:01

Biology, 12.10.2020 19:01

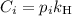
 is the partial pressure of the gas.
is the partial pressure of the gas.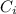 is the concentration of the gas (solubility).
is the concentration of the gas (solubility). is Henry's constant, which depends on the nature of the gas, the temperature and the liquid.
is Henry's constant, which depends on the nature of the gas, the temperature and the liquid.