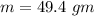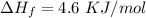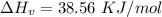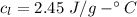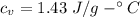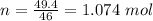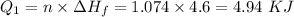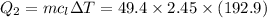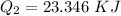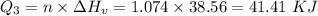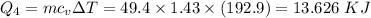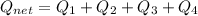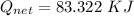Physics, 08.01.2020 02:31 bwright142
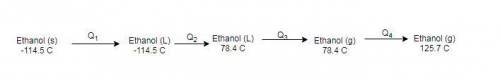
How much heat energy is required to convert 49.4 g of solid ethanol at − 114.5 ° c to gasesous ethanol at 140.7 ° c ? the molar heat of fusion of ethanol is 4.60 kj/mol , and its molar heat of vaporization is 38.56 kj/mol . ethanol has a normal melting point of − 114.5 ° c and a normal boiling point of 78.4 ° c . the specific heat capacity of liquid ethanol is 2.45 j / g ⋅ ° c , and that of gaseous ethanol is 1.43 j / g ⋅ ° c .

Answers: 3


Another question on Physics

Physics, 21.06.2019 20:30
Protons and neutrons are found within the nucleus of an atom
Answers: 2

Physics, 22.06.2019 00:50
Part a constants huck finn walks at a speed of 0.60 m/s across his raft (that is, he walks perpendicular to the raft's motion what is huck's velocity (speed and direction) relative to the river bank? express your answer to three significant figures and include the appropriate units. relative to the shore)
Answers: 3

Physics, 22.06.2019 12:00
Selma made a diagram to compare convection and radiation. which label belongs in the area marked x? must involve temperature differences between substances or objects only occurs when molecules are in direct contact involves the movement of fluids based on density differences can occur where there is little or no matter
Answers: 1

Physics, 22.06.2019 17:50
If there's a small amount of friction between two surfaces, the result could be select all that applya. no movement b. heatc. a little bit of movementd. sliding around
Answers: 2
You know the right answer?
How much heat energy is required to convert 49.4 g of solid ethanol at − 114.5 ° c to gasesous ethan...
Questions




Biology, 05.05.2020 05:03





Mathematics, 05.05.2020 05:03


Mathematics, 05.05.2020 05:03

Chemistry, 05.05.2020 05:03




Geography, 05.05.2020 05:03

English, 05.05.2020 05:03


English, 05.05.2020 05:03