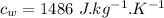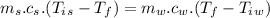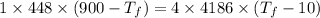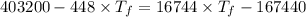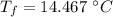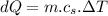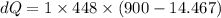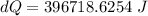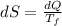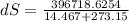Physics, 24.12.2019 06:31 haleyrene3924
A1.00-kg iron horseshoe is taken from a forge at 900∘c and dropped into 4.00 kg of water at 10.0∘c. assuming that no energy is lost by heat to the surroundings, determine the total entropy change of the horseshoe-plus-water system.

Answers: 1


Another question on Physics


Physics, 22.06.2019 18:50
An insulated thermos contains 148 g of water at 72.7 ˚c. you put in a 11.7 g ice cube at 0.00 ˚c to form a system of ice + original water. the specific heat of liquid water is 4190 j/kg•k; and the heat of fusion of water is 333 kj/kg. what is the net entropy change of the system from then until the system reaches the final (equilibrium) temperature?
Answers: 2

Physics, 23.06.2019 01:00
The receptor cells that convert light energy into neural signals are called
Answers: 2

Physics, 23.06.2019 01:30
Listed following are various physical situations that describe how light interacts with matter. match these to the appropriate category. 1) transmission? -visible light meets clear glass -visible light does not pass through a black wall -red light hits a red sweatshirt -light comes from your computer screen -cell phone signals pass through walls -blue light hits a red sweatshirt -white light hits a white piece of paper -light comes from a light bulb
Answers: 1
You know the right answer?
A1.00-kg iron horseshoe is taken from a forge at 900∘c and dropped into 4.00 kg of water at 10.0∘c....
Questions

Mathematics, 13.11.2020 18:30

Biology, 13.11.2020 18:30


English, 13.11.2020 18:30

English, 13.11.2020 18:30

English, 13.11.2020 18:30

Mathematics, 13.11.2020 18:30

Mathematics, 13.11.2020 18:30


English, 13.11.2020 18:30

Mathematics, 13.11.2020 18:30




Mathematics, 13.11.2020 18:30

Chemistry, 13.11.2020 18:30

Arts, 13.11.2020 18:30

Business, 13.11.2020 18:30

Biology, 13.11.2020 18:30

Biology, 13.11.2020 18:30

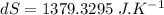
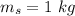 initial temperature of iron,
initial temperature of iron, 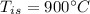 mass of water,
mass of water, 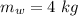 initial temperature of water,
initial temperature of water, 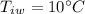
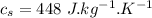 Specific heat of water,
Specific heat of water,