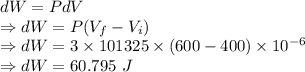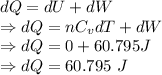An ideal gas expands at a constant total pressure of 3.0 atm from 400 ml to 600 ml. heat then flows out of the gas at a constant volume, and the pressure and temperature are allowed to drop until the temperature reaches its original value. calculate: (a) the total work done by the gas in the process.(b) the total heat flow into the gas.

Answers: 3


Another question on Physics

Physics, 22.06.2019 01:00
15. give an example for some particles or waves that are moving faster than light in everyday life 16. what is a laser? 17. what is an oscilloscope? 18. what does it means practically that nothing is faster than light in vacuum? 19. what is vacuum?
Answers: 2

Physics, 22.06.2019 07:00
We put a force of 50n on an object and the acceleration is 100 m/s². what is the mass of the object?
Answers: 1

Physics, 22.06.2019 07:40
An object's buoyant force and weight mean the same thing.a. trueb. false
Answers: 2

Physics, 22.06.2019 09:00
Chemical energy is a form of energy. a. heat b. kinetic c. potential d. electromagnetic
Answers: 2
You know the right answer?
An ideal gas expands at a constant total pressure of 3.0 atm from 400 ml to 600 ml. heat then flows...
Questions

Mathematics, 12.10.2021 07:40


Mathematics, 12.10.2021 07:40

Mathematics, 12.10.2021 07:40

History, 12.10.2021 07:40


Mathematics, 12.10.2021 07:40

Mathematics, 12.10.2021 07:40

History, 12.10.2021 07:40



Mathematics, 12.10.2021 07:40

English, 12.10.2021 07:40





Mathematics, 12.10.2021 07:40


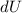 = Internal energy =
= Internal energy = 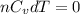
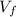 = Final volume = 600 mL
= Final volume = 600 mL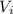 = Initial volume = 400 mL
= Initial volume = 400 mL