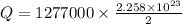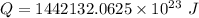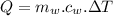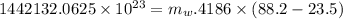Consider the following thermochemical equation:
2ch3oh(l) + 3o2(g) → 2co2(g) + 4h2o(g) ∆horx...

Physics, 21.12.2019 02:31 magalyrodette7812
Consider the following thermochemical equation:
2ch3oh(l) + 3o2(g) → 2co2(g) + 4h2o(g) ∆horxn = –1277 kj
what mass of water can be heated from 23.5°c to 88.2°c with the heat that is released from the combustion of 12.0 g of ch3oh (molar mass = 32.0 g/mol), assuming no heat is lost to the surroundings? specific heat capacity of h2o = 4.184 j/g°c.

Answers: 2


Another question on Physics

Physics, 22.06.2019 07:00
Hen a gfci receptacle device is installed on a 20-ampere branch circuit (12 awg copper), what is the minimum volume allowance (in cubic inches) required for conductor fill for each conductor in the outlet box?
Answers: 1

Physics, 22.06.2019 07:30
Clothes dryer uses about 7 amps of current from a 240 volt line. how much power does it use?
Answers: 1

Physics, 22.06.2019 08:40
The system is released from rest with the cable taut, and the homogeneous cylinder does not slip on the rough incline. determine the angular acceleration of the cylinder and the minimum coeffi cient s of friction for which the cylinder will not slip.
Answers: 2

Physics, 22.06.2019 11:50
The electric field between square plates of a parallel-plate capacitor has magnitude e. the potential across the plates is maintained with constant voltage by a battery as they are pulled apart to twice their original separation, which is small compared to the dimensions of the plates. the magnitude of the electric field between the plates is now equal to a)e b)e/4 c)e/2 d)4e e)2e
Answers: 1
You know the right answer?
Questions




Mathematics, 07.03.2021 23:50


Mathematics, 07.03.2021 23:50

Spanish, 07.03.2021 23:50

Mathematics, 07.03.2021 23:50


Mathematics, 07.03.2021 23:50

English, 07.03.2021 23:50

Mathematics, 07.03.2021 23:50

Social Studies, 07.03.2021 23:50


Mathematics, 07.03.2021 23:50


Mathematics, 07.03.2021 23:50

English, 07.03.2021 23:50

Mathematics, 07.03.2021 23:50

Mathematics, 07.03.2021 23:50

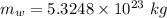
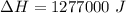
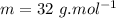
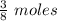
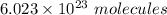
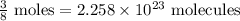
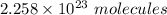 :
: