Physics, 20.12.2019 19:31 unicornpoop54
An 80.0-g piece of copper, initially at 295°c, is dropped into 250 g of water contained in a 300-g aluminum calorimeter; the water and calorimeter are initially at 10.0°c.
what is the final temperature of the system? (specific heats of copper and aluminum are 0.092 0 and 0.215 cal/g⋅°c, respectively. cw = 1.00 cal/g°c)
a. 12.8°c
b. 16.5°c
c. 28.4°c
d. 32.1°c

Answers: 2


Another question on Physics

Physics, 22.06.2019 02:00
The image shows a pendulum in simple harmonic motion the pendulum starts at a and swing to e
Answers: 1

Physics, 22.06.2019 10:30
Awoman holds a book by placing it between her hands such that she presses at right angles to the front and back covers. the book has a mass of m = 1.8 kg and the coefficient of static friction between her hand and the book is μs = 0.67. no attempt 50% part (a) what is the weight of the book, fgb in newtons?
Answers: 3


Physics, 22.06.2019 13:10
Most short-period comets do not have randomly oriented orbits because
Answers: 2
You know the right answer?
An 80.0-g piece of copper, initially at 295°c, is dropped into 250 g of water contained in a 300-g a...
Questions

Biology, 31.08.2019 03:30




Mathematics, 31.08.2019 03:30

Mathematics, 31.08.2019 03:30


Biology, 31.08.2019 03:30

History, 31.08.2019 03:30

History, 31.08.2019 03:30

Social Studies, 31.08.2019 03:30




Mathematics, 31.08.2019 03:30


Mathematics, 31.08.2019 03:30


English, 31.08.2019 03:30

Geography, 31.08.2019 03:30

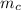 = mass of piece of copper = 80 g
= mass of piece of copper = 80 g 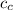 = specific heat of piece of copper = 0.0920 cal/g°C
= specific heat of piece of copper = 0.0920 cal/g°C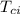 = Initial temperature of piece of copper = 295 °C
= Initial temperature of piece of copper = 295 °C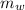 = mass of water = 250 g
= mass of water = 250 g 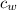 = specific heat of water = 1 cal/g°C
= specific heat of water = 1 cal/g°C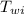 = Initial temperature of piece of copper = 10 °C
= Initial temperature of piece of copper = 10 °C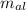 = mass of calorimeter = 300
= mass of calorimeter = 300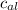 = specific heat of calorimeter = 0.215 cal/g°C
= specific heat of calorimeter = 0.215 cal/g°C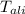 = Initial temperature of calorimeter = 10 °C
= Initial temperature of calorimeter = 10 °C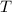 = Final equilibrium temperature
= Final equilibrium temperature