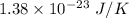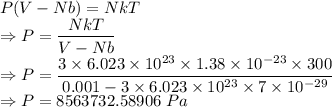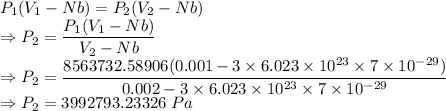Physics, 19.12.2019 23:31 bobtothemaxthe1st
Compressed gases aren't ideal. let's consider a gas that's non-ideal only because the volume available to each of the n molecules is reduced because each other molecule occupies volume v. instead of pv=nkt, we get: p(v-nb)=nkt. let b=7 × 10^-29 m3. let's look at 3 moles of this gas at t=300k starting in 0.001 m3 volume.
1) what's the initial value of the pressure? p_initial = ?
2) the gas expands isothermally to 0.002 m3. what's the final pressure? p_final = ?
3) how much work did the gas do in this isothermal expansion? w = ?

Answers: 1


Another question on Physics

Physics, 21.06.2019 19:10
Athin, square metal plate measures 14 cm on each side and has emissivity of 0.60. the plate is heated to a temperature of 745°c. what is the rate at which the plate radiates energy ? the stefan-boltzmann constant is 5.67 × 10-8 w/(m2 ? k4). remember that the plate will radiate energy from both its top and bottom surfaces.
Answers: 1

Physics, 22.06.2019 00:30
Asap time is ! best answer gets compose at least one well-developed paragraph on the following: define the term concurrent powers, and give an example of a concurrent power of government.
Answers: 1


Physics, 22.06.2019 14:40
According to valence bond theory, which orbitals overlap in the formation of the bond in hf according to valence bond theory, which orbitals overlap in the formation of the bond in hf 2s on h and 2p on f 1s on h and 2s on f 1s on h and 1p on f 1s on h and 2p on f 1s on h and 3p on f
Answers: 3
You know the right answer?
Compressed gases aren't ideal. let's consider a gas that's non-ideal only because the volume availab...
Questions

English, 16.11.2020 08:30


Mathematics, 16.11.2020 08:30

Mathematics, 16.11.2020 08:30

Chemistry, 16.11.2020 08:30












Mathematics, 16.11.2020 08:40



Mathematics, 16.11.2020 08:40

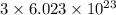
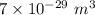
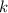 = Boltzmann constant =
= Boltzmann constant =