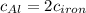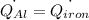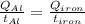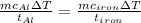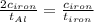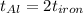Physics, 18.12.2019 22:31 Amaris0901
The specific heat capacity of aluminum is about twice that of iron. consider two blocks of equal mass, one made of aluminum and the other one made of iron, initially in thermal equilibrium.
heat is added to each block at the same constant rate until it reaches a temperature of 500 k. which of the following statements is true?
a. the iron takes less time than the aluminum to reach the final temperature.
b. the aluminum takes less time than the iron to reach the final temperature.
c. the two blocks take the same amount of time to reach the final temperature.

Answers: 1


Another question on Physics

Physics, 22.06.2019 05:30
Which of the following are considered noble gases? a. bromine b. neon c. argon d. chlorine
Answers: 1

Physics, 22.06.2019 06:00
Why don't you see your reflection in water with waves or repples
Answers: 1

Physics, 22.06.2019 07:00
If a tank filled with water contains a block and the height of the water above point a within the block is 0.8 meter, what is the pressure at point a?
Answers: 2

Physics, 22.06.2019 10:00
If a stone with an original velocity of 0 is falling from a ledgeand takes 8 seconds to hoybthe ground whays the final velocity of the stone
Answers: 2
You know the right answer?
The specific heat capacity of aluminum is about twice that of iron. consider two blocks of equal mas...
Questions


Mathematics, 04.12.2020 01:00

Social Studies, 04.12.2020 01:00


Mathematics, 04.12.2020 01:00

Mathematics, 04.12.2020 01:00



Mathematics, 04.12.2020 01:00




Chemistry, 04.12.2020 01:00


Arts, 04.12.2020 01:00


Mathematics, 04.12.2020 01:00

Mathematics, 04.12.2020 01:00