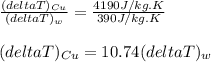Physics, 18.12.2019 03:31 7thaohstudent
A1.0 kg piece of copper with a specific heat of ccu=390j/(kg⋅k) is placed in 1.0 kg of water with a specific heat of cw=4190j/(kg⋅k). the copper and water are initially at different temperatures. after a sufficiently long time, the copper and water come to a final equilibrium temperature. part a which of the following statements is correct concerning the temperature changes of both substances? (ignore the signs of the temperature changes in your answer.) which of the following statements is correct concerning the temperature changes of both substances? (ignore the signs of the temperature changes in your answer.) the temperature change of the copper is equal to the temperature change of the water. the temperature change of the water is greater than the temperature change of the copper. the temperature change of the copper is greater than the temperature change of the water.

Answers: 2


Another question on Physics

Physics, 21.06.2019 15:00
Air flows upward in the wick of a lantern because of the liquid property called
Answers: 1

Physics, 21.06.2019 20:00
Excuse ! extra points plus ! super desperate ! the position of an object does not move relative to a reference point. relative to the reference point the object _. a.) is not moving. b.) is moving.
Answers: 1

Physics, 22.06.2019 05:00
Which is the best predictor of the radioactive nature of an isotope? o the proton-to-electron ratio the neutron-to-proton ratio o the neutron-to-electron ratio the electron-to-proton ratio
Answers: 1

Physics, 22.06.2019 14:00
Acar travels in reverse. it covers 150 meters in 12 seconds what is the velocity of the car what is the speed of the car
Answers: 1
You know the right answer?
A1.0 kg piece of copper with a specific heat of ccu=390j/(kg⋅k) is placed in 1.0 kg of water with a...
Questions


English, 10.07.2019 20:00

English, 10.07.2019 20:00




Mathematics, 10.07.2019 20:00

Social Studies, 10.07.2019 20:00

Health, 10.07.2019 20:00

English, 10.07.2019 20:00


Physics, 10.07.2019 20:00


Geography, 10.07.2019 20:00


Spanish, 10.07.2019 20:00

Mathematics, 10.07.2019 20:00



Mathematics, 10.07.2019 20:00