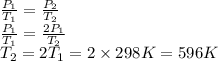Suppose a sample of an ideal gas in a container is subjected to a temperature change. a decrease in temperature will the kinetic energy and average speed of the gas particles. as a result, the pressure on the walls of the container will if the gas starts at 25 ∘ c, what temperature would the gas need to reach for its pressure to double? temperature = ∘ c

Answers: 1


Another question on Physics

Physics, 22.06.2019 10:30
Agroup of students were investigating the force of gravity. they began by dropping a foam ball from a height of 3 meters into a bucket of sand. the ball hit the sand in 0.306 seconds. they dropped additional balls of approximately the same diameter, but of different masses. here is the data they collected. based on this experiment and the collected data, what would their conclusion be?
Answers: 1

Physics, 22.06.2019 11:20
How to tell if a molecule is polar or nonpolar with electronegativity
Answers: 2

Physics, 22.06.2019 17:10
What causes the development of most clouds and precipitation in the atmosphere?
Answers: 1

Physics, 22.06.2019 20:10
Consider two less-than-desirable options. in the first you are driving 30 mph and crash head-on into an identical car also going 30 mph. in the second option you are driving 30 mph and crash head-on into a stationary brick wall. in neither case does your car bounce off the thing it hits, and the collision time is the same in both cases. which of these two situations would result in the greatest impact force?
Answers: 1
You know the right answer?
Suppose a sample of an ideal gas in a container is subjected to a temperature change. a decrease in...
Questions

Health, 02.10.2019 03:40




History, 02.10.2019 03:40



History, 02.10.2019 03:40

English, 02.10.2019 03:40





Chemistry, 02.10.2019 03:40



Mathematics, 02.10.2019 03:40

Mathematics, 02.10.2019 03:40

Chemistry, 02.10.2019 03:40

History, 02.10.2019 03:40