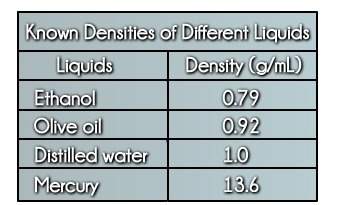
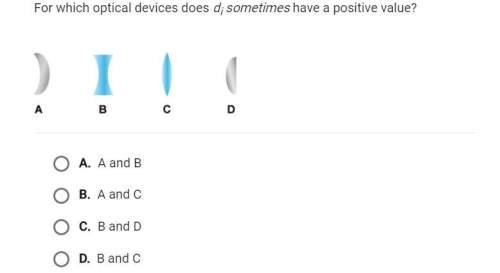
Physics, 17.12.2019 20:31 madelyngv97
Suppose you have a liquid in equilibrium with its gas phase inside a closed container that is in contact witha thermal reservoir. you then pump in an inert gas raising the pressure exerted on the liquid. does thefraction of the liquid in the vapor phase decrease or increase? a) use the definition of the gibbs free energy in terms of the chemical potential and the assumption that thevapor phase is an ideal gas to show that the chemical potential depends on the pressure in the following way: pμ(t, p) = μ(t, p 0) + kb t ln, p 0where p is the total pressure and p 0 is the initial vapor pressure in the absence of the added inert gas.

Answers: 2


Another question on Physics

Physics, 21.06.2019 19:00
Many machines- including inclined planes such as ramps- increase the strength of the force put into the machine but decrease the distance over which the force is applied. true or false
Answers: 1

Physics, 22.06.2019 15:00
You hang a book bag on a spring scale and place the bag on a platform scale so that the platform scale reads 23.7n and the spring scale reads 13.8n. what is the magnitude of the force that earth exerts on the bag?
Answers: 1

Physics, 22.06.2019 17:40
Aball is thrown horizontally from a cliff at 8m/s .in what direction is the ball moving 2s later?
Answers: 1

Physics, 22.06.2019 18:30
4. now look at the green lines you created by connecting the three boiling point data points and the three melting point data points. for each of these lines, describe any trends you see. 5. locate the elements on your periodic table that you circled in green on your graph. what term or description would you use to identify these elements with respect to the periodic table? 7. using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers.
Answers: 2
You know the right answer?
Suppose you have a liquid in equilibrium with its gas phase inside a closed container that is in con...
Questions

Business, 15.10.2019 06:30


Mathematics, 15.10.2019 06:30

Physics, 15.10.2019 06:30



History, 15.10.2019 06:30



Spanish, 15.10.2019 06:30



Mathematics, 15.10.2019 06:30






Mathematics, 15.10.2019 06:30