Physics, 17.12.2019 04:31 mrflexington77
600-g of ice at −15℃ is in a calorimeter when 100 g of water at 20℃ is added to it. water cools down and part of it freezes as a result. find the mass of the remaining liquid water. neglect the heat capacity of the calorimeter. [ = 0.5 cal/g/℃ = 2090 j/kg/℃,
= 0.5 cal/g/℃ = 2090 j/kg/℃,  = 1 cal/g/℃ = 4186j/kg/℃,
= 1 cal/g/℃ = 4186j/kg/℃,  = 79.7 cal/g = 3.33 × 10⁵ j/kg]
= 79.7 cal/g = 3.33 × 10⁵ j/kg]

Answers: 2


Another question on Physics

Physics, 22.06.2019 09:00
What is a possible result of higher air temperature caused by global warming
Answers: 1

Physics, 22.06.2019 11:30
What is the name for the remnant of an asymptotic giant that has lost its shells? black dwarf white dwarf yellow giant black hole
Answers: 3

Physics, 22.06.2019 15:20
Abag of potato chips contains 2.00 l of air when it is sealed at sea level at a pressure of 1.00 atm and a temperature of 20.0 deg c. what will be the volume of the air in the bag if you take it with you, still sealed, to the mountains where the temperature is 7.00 deg c and atmospheric pressure is 70.0 kpa? assume that the bag behaves like a balloon and that the air in the bag is in thermal equilibrium with the outside air.
Answers: 3

Physics, 22.06.2019 22:50
Which lists correctly orders nuclear reactions from most radioactive waste generated to least waste generated within a given period of time? o a. radioactive decay, nuclear fission, nuclear fusion o b. radioactive decay, nuclear fusion, nuclear fission o c. nuclear fusion, nuclear fission, radioactive decay o d. nuclear fission, nuclear fusion, radioactive decay
Answers: 3
You know the right answer?
600-g of ice at −15℃ is in a calorimeter when 100 g of water at 20℃ is added to it. water cools down...
Questions


Biology, 02.12.2021 01:00


Mathematics, 02.12.2021 01:00






Computers and Technology, 02.12.2021 01:00

English, 02.12.2021 01:00





English, 02.12.2021 01:00

English, 02.12.2021 01:00

Health, 02.12.2021 01:00

Mathematics, 02.12.2021 01:00

Mathematics, 02.12.2021 01:00

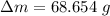
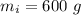 initial temperature of ice,
initial temperature of ice, 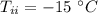 mass of water added,
mass of water added, 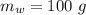 initial temperature of water,
initial temperature of water, 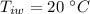 specific heat capacity of ice,
specific heat capacity of ice, 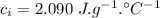 specific heat capacity of water,
specific heat capacity of water, 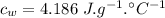 latent heat of fusion,
latent heat of fusion, 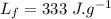
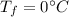
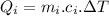
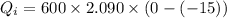
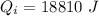 .....................................(1)
.....................................(1)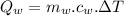
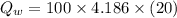
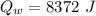 ...............................................(2)
...............................................(2)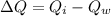
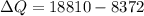
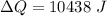
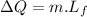
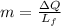
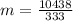
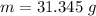 of more ice is formed from the water.
of more ice is formed from the water.