
At 250 °c, the equilibrium constant kp for the reaction pcl5 (g) pcl3 (g) + cl2 (g) is 1.80. sufficient pcl5 is put into a reaction vessel to give an initial pressure of 2.74 atm at 250 °c. calculate the pressure of pcl5 after the system has reached equilibrium.
a. 1.50 atm
b. 1.24 atm
c. 4.24 atm
d. 0.94 atm
e. 1.12 atm

Answers: 3


Another question on Physics

Physics, 21.06.2019 14:10
Gam radio signals can usually be heard behind a hill, but fm often cannot. that is, am signals bend more than fm. explain. (radio signals, as we shall see, are carried by electromagnetic waves whose wavelength for am is typically 200 to 600 m and for fm about 3 m.)
Answers: 2

Physics, 21.06.2019 18:50
The summit of chimborazo, in ecuador, which is at a distance of about 6,384 km from the earth's center. the bottom of the mariana trench, in the western pacific ocean, which is nearly 6,370 km from the center of the earth. on the surface of earth on the equator line.
Answers: 1

Physics, 21.06.2019 20:30
When a book falls from a shelf to the ground without anyone pushing on it, no work is done on the book. true or false
Answers: 1

Physics, 22.06.2019 07:20
Aman throws a football straight into the air. as it rises, it slows down. which type of energy is the football gaining?
Answers: 2
You know the right answer?
At 250 °c, the equilibrium constant kp for the reaction pcl5 (g) pcl3 (g) + cl2 (g) is 1.80. suffici...
Questions

Mathematics, 31.03.2020 18:48



Mathematics, 31.03.2020 18:48

Mathematics, 31.03.2020 18:48



Mathematics, 31.03.2020 18:49

Mathematics, 31.03.2020 18:49


Mathematics, 31.03.2020 18:49



History, 31.03.2020 18:49



Mathematics, 31.03.2020 18:49

Mathematics, 31.03.2020 18:49

Mathematics, 31.03.2020 18:49

History, 31.03.2020 18:49

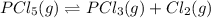
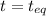 2.74-x x x
2.74-x x x
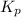 for the given reaction follows:
for the given reaction follows:
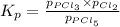
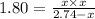
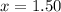
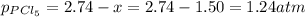
 at equilibrium is 1.24 atm
at equilibrium is 1.24 atm

