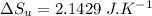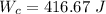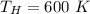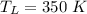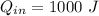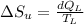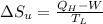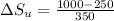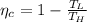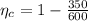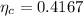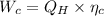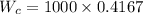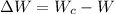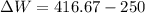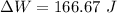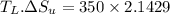Physics, 16.12.2019 23:31 reghanhoward3
1. a heat engine operates between two reservoirs at t2 = 600 k and t1 = 350 k. it takes in 1.00 x 103 j of energy from the higher-temperature reservoir and performs 250 j of work. find a. the entropy change of the universe δsu for this process and b. the work w that could have been done by an ideal carnot engine operating between these two reservoirs. c. show that the difference between the amounts of work done in parts (a) and (b) is t1δsu.

Answers: 1


Another question on Physics

Physics, 21.06.2019 16:10
In 1995 a research group led by eric cornell and carl wiemann at the university of colorado successfully cooled rubidium atoms to the 20-200 nk temperature range. assuming (incorrectly) that the rubidium atoms behave liké particles of a classical ideal gas, calculate the rms speed of a rubidium atom at a temperature of 112.0 nk. in the experiments one particular isotope of rubidium was used, rubidium-87. the molar mass of this isotope is 86.91 q/mol. tries 0/20 submit answer
Answers: 1

Physics, 21.06.2019 19:30
The 20@kg wheel has a radius of gyration about its center g of kg = 300 mm. when it is subjected to a couple moment of m = 50 n # m, it rolls without slipping. determine the angular velocity of the wheel after its mass center g has traveled through a distance of sg = 20 m, starting from rest.
Answers: 3

Physics, 21.06.2019 22:40
Explain vector addition, triangle method and parallelogram method
Answers: 1

You know the right answer?
1. a heat engine operates between two reservoirs at t2 = 600 k and t1 = 350 k. it takes in 1.00 x 10...
Questions

Mathematics, 04.05.2021 05:20

Mathematics, 04.05.2021 05:20





Computers and Technology, 04.05.2021 05:20

Social Studies, 04.05.2021 05:20




Mathematics, 04.05.2021 05:20

Mathematics, 04.05.2021 05:20

Biology, 04.05.2021 05:20


English, 04.05.2021 05:20

History, 04.05.2021 05:20

Mathematics, 04.05.2021 05:20


Mathematics, 04.05.2021 05:20