
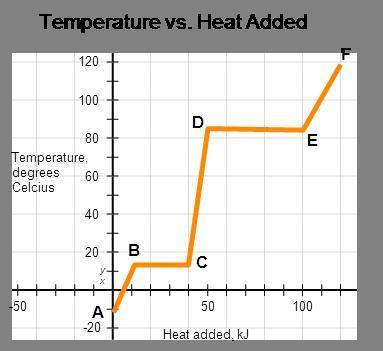
The represented substance has the following heat capacities, enthalpy of fusion, and enthalpy of vaporization.
cp, solid = 3.2 j/(g•°c); cp, liquid = 5.3 j/(g•°c);
cp, vapor = 8.9 j/(g•°c); δhfus = 4.5 kj/mol;
δhvap = 8.6 kj/mol
calculate the total energy input required to accomplish vaporization of one mole of substance at 15°c. the molar mass of the substance is 120.0 g/mol.

Answers: 2


Another question on Physics

Physics, 21.06.2019 22:30
How have the competing explanations' experiments on atoms affected the development of the atomic model?
Answers: 1

Physics, 22.06.2019 07:30
Which of the following is an example of motion in two dimensions?
Answers: 3

Physics, 22.06.2019 07:30
Gas cloud 1 is likely to form a star. gas cloud 2 is not. based on this information, match the given conditions with each cloud
Answers: 2

Physics, 22.06.2019 21:30
Calculate the minimum energy required to remove one proton from the nucleus 126c. this is called the proton-removal energy. (hint: find the difference between the mass of a 126c nucleus and the mass of a proton plus the mass of the nucleus formed when a proton is removed from 126c.) express your answer with the appropriate units. emin e m i n = nothing nothing request answer part b how does the proton-removal energy for 126c compare to the binding energy per nucleon for 126c, calculated using eb=(zmh+nmn−azm)c2?
Answers: 1
You know the right answer?
The represented substance has the following heat capacities, enthalpy of fusion, and enthalpy of vap...
Questions




Mathematics, 05.11.2020 19:00



Mathematics, 05.11.2020 19:00

Spanish, 05.11.2020 19:00







Mathematics, 05.11.2020 19:00


Mathematics, 05.11.2020 19:00

Mathematics, 05.11.2020 19:00

English, 05.11.2020 19:00



