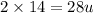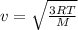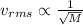Physics, 16.12.2019 18:31 neylabaker7489
The atomic mass of a nitrogen atom (n) is 14.0 u, while that of an oxygen atom (o) is 16.0 u. three diatomic gases have the same temperature: nitrogen (n2), oxygen (o2), and nitric oxide (no). rank these gases in ascending order (smallest first), according to the values of their translational rms speeds.
a. n2, o2, no
b. o2, no, n2
c. o2, n2, no
d. no, n2, o2

Answers: 1


Another question on Physics

Physics, 22.06.2019 10:00
Which atomic model was proposed as a result of j. j. thomson’s work?
Answers: 1

Physics, 22.06.2019 13:10
Which of the following are used in an electrochemical cell? a. electrodes b. electrolyte c. terminals d. all of the above
Answers: 3

Physics, 22.06.2019 22:00
It takes a lot of energy to get the temperature of water to increase and eventually boil because water has a high heat.
Answers: 1

Physics, 23.06.2019 01:00
The bubbles in a carbonated soft drink are produced when carbonic acid decomposes to form carbon dioxide and water. in a closed system, this equilibrium exists as why does a carbonated soft drink lose carbonation when the container is left open? a.water evaporates, which favors the formation of h2co3. b.pressure decreases, which favors the formation of h2co3. c.the open container warms up, which causes more gas to escape. d.co2is removed, which favors the formation of h2co3.
Answers: 2
You know the right answer?
The atomic mass of a nitrogen atom (n) is 14.0 u, while that of an oxygen atom (o) is 16.0 u. three...
Questions



Mathematics, 05.05.2020 22:45

Mathematics, 05.05.2020 22:45


History, 05.05.2020 22:45



History, 05.05.2020 22:45

History, 05.05.2020 22:45

Mathematics, 05.05.2020 22:45



Business, 05.05.2020 22:45

History, 05.05.2020 22:45

Mathematics, 05.05.2020 22:45

English, 05.05.2020 22:45

Mathematics, 05.05.2020 22:45


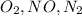
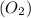 =
=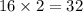 u
u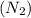 =
=