
Physics, 13.12.2019 04:31 kmoo176394
Amonatomic ideal gas that is initially at a pressure of 1.50 x 10^5 pa and has a volume of 0.08 m^3 is compressed adiabatically to a volume of 0.0400 m^3.
(a) what is the final pressure?
(b) how much work is done by the gas?
(c) what is the ratio of the final temperature of the gas to its initial temperature? is the gas heated or cooled by this compression?

Answers: 3


Another question on Physics

Physics, 22.06.2019 13:00
Discuss how the hardness or softness of the landing surface is related to the time required to stop the egg
Answers: 1

Physics, 22.06.2019 14:00
Una carga puntual de 3 x 10-6 c se coloca a 12 cm de una segunda carga puntual de - 1,5 x 10-6 c. calcular la magnitud fuerza eléctrica entre las cargas
Answers: 2

Physics, 22.06.2019 16:00
While the change in blank will remain the same during a collision, the force needed to bring an object to a stop can be blank if the time if collision is blank
Answers: 1

Physics, 22.06.2019 17:20
In a system with only a single force acting upon a body, what is the relationship between the change in kinetic energy and the work done by the force? answers: work is equal to the change in kinetic energy.work depends on the square of the change in potential energy.work is equal to the negative of the change in kinetic energy.work is equal to the square of the change in kinetic energy
Answers: 2
You know the right answer?
Amonatomic ideal gas that is initially at a pressure of 1.50 x 10^5 pa and has a volume of 0.08 m^3...
Questions

Mathematics, 28.10.2020 17:00


Mathematics, 28.10.2020 17:00

Mathematics, 28.10.2020 17:00









History, 28.10.2020 17:00

History, 28.10.2020 17:00


Mathematics, 28.10.2020 17:00




History, 28.10.2020 17:00

 .
.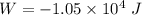 .
.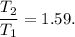 and gas is heated.
and gas is heated.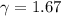 .
. .
.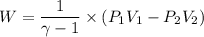 .
. .
.

