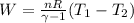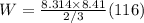Answers: 1


Another question on Physics

Physics, 21.06.2019 20:00
Spend time observing or thinking about events that involve matter and energy. which events can you explain? which events can’t you explain?
Answers: 1

Physics, 22.06.2019 08:30
A17,250 kg rocket is pushed with a thrust of 6,450,284 n. what is the acceleration of the rocket?
Answers: 1


Physics, 22.06.2019 13:00
The magnitude of the amount of energy released by burning a fuel source, measured in energy per unit mass, is called its fuel value. note that the fuel value is the negative of the isobaric specific heat of combustion for the fuel. if all the energy obtained from burning 1.23 pounds of butane with a fuel value of 10.85 kcal/g is used to heat 128.0 kg of water at an initial temperature of 18.3 °c, what is the final temperature? note that 1 lb = 453.6 g.
Answers: 3
You know the right answer?
Suppose 8.41 moles of a monatomic ideal gas expand adiabatically, and its temperature decreases from...
Questions

Mathematics, 23.06.2019 06:30

Mathematics, 23.06.2019 06:30

Mathematics, 23.06.2019 06:30







English, 23.06.2019 06:30






Mathematics, 23.06.2019 06:30


English, 23.06.2019 06:30

English, 23.06.2019 06:30