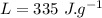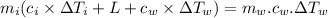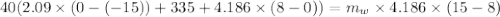A40.0-g block of ice at -15.00°c is dropped into a calorimeter (of negligible heat capacity) containing water at 15.00°c.
when equilibrium is reached, the final temperature is 8.00°c.
how much water did the calorimeter contain initially?
the specific heat of ice is 2090 j/kg • k, that of water is 4186 j/kg • k, and the latent heat of fusion of water is 33.5 × 104 j/kg.

Answers: 1


Another question on Physics

Physics, 21.06.2019 18:00
Which surface feature of the moon is characterized by mountainous areas? terrae craters maria regolith
Answers: 1

Physics, 21.06.2019 19:10
Natural forces that can alter ecosystems include seasons and climate changes. t or f
Answers: 1

Physics, 22.06.2019 10:00
(a) calculate the number of electrons in a small, electrically neutral silver pin that has a mass of 10.0 g. silver has 47 electrons per atom, and its molar mass is 107.87 g/mol. (b) imagine adding electrons to the pin until the negative charge has the very large value 1.00 mc. how many electrons are added for every 109 electrons already present
Answers: 3

Physics, 22.06.2019 12:50
The combining of light nuclei is called blank. blank as in not actually blank. you know what im tryin to say.
Answers: 1
You know the right answer?
A40.0-g block of ice at -15.00°c is dropped into a calorimeter (of negligible heat capacity) contain...
Questions



Biology, 05.07.2019 15:10

History, 05.07.2019 15:10



Biology, 05.07.2019 15:10

History, 05.07.2019 15:10


Mathematics, 05.07.2019 15:10

Mathematics, 05.07.2019 15:10

History, 05.07.2019 15:10

Biology, 05.07.2019 15:10




Mathematics, 05.07.2019 15:10

History, 05.07.2019 15:10


Mathematics, 05.07.2019 15:10

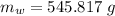
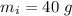 initial temperature of ice block,
initial temperature of ice block,  initial temperature of water,
initial temperature of water,  final temperature of mixture,
final temperature of mixture, 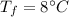 specific heat of ice,
specific heat of ice, 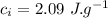 specific heat of water,
specific heat of water, 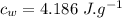 Latent heat of fusion of water,
Latent heat of fusion of water,