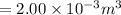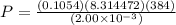Physics, 09.12.2019 23:31 Jacobstoltzfus
Acook puts 1.90 g of water in a 2.00 l pressure cooker that is then warmed from the kitchen temperature of 20°c to 111°c. what is the pressure (in atm) inside the container?

Answers: 2


Another question on Physics

Physics, 22.06.2019 00:30
Consider an ordinary, helium-filled party balloon with a volume of 2.2 ft3. the lifting force on the balloon due to the outside air is the net resultant of the pressure distribution exerted on the exterior surface of the balloon. using this fact, we can derive archimedes’ principle, namely that the upward force on the balloon is equal to the weight of the air displaced by the balloon. assuming that the balloon is at sea level, where the air density is 0.002377 slug/ft3, calculate the maximum weight that can be lifted by the balloon. note: the molecular weight of air is 28.8 and that of helium is 4.
Answers: 2

Physics, 22.06.2019 11:00
1. jay fills a wagon with sand (about 20 kg) and pulls it with a rope 30 m along the beach. he holds the rope 25° above the horizontal. the rope exerts a 20-n tension force on the wagon. how much work does the rope do on the wagon?
Answers: 1

Physics, 22.06.2019 12:50
Air is contained in a variable-load piston-cylinder device equipped with a paddle wheel. initially, air is at 400 kpa and 17°c. the paddle wheel is now turned by an external electric motor until 75 kj/kg of work has been transferred to air. during this process, heat is transferred to maintain a constant air temperature while allowing the gas volume to triple. calculate the required amount of heat transfer in kj/kg.
Answers: 2

Physics, 22.06.2019 15:00
Mechanical energy is when the amount of kinetic and potential energies added together remains the same. conserved created lost
Answers: 1
You know the right answer?
Acook puts 1.90 g of water in a 2.00 l pressure cooker that is then warmed from the kitchen temperat...
Questions


Social Studies, 15.12.2020 23:00


Advanced Placement (AP), 15.12.2020 23:00

Mathematics, 15.12.2020 23:00

Biology, 15.12.2020 23:00

Physics, 15.12.2020 23:00

Mathematics, 15.12.2020 23:00

Mathematics, 15.12.2020 23:00

Mathematics, 15.12.2020 23:00


History, 15.12.2020 23:00




Physics, 15.12.2020 23:00




Chemistry, 15.12.2020 23:00