
Physics, 05.12.2019 03:31 lexhorton2002
The combined statement of the first and second laws for the change in enthalpy of unary single-phase system may be written: dh' = tds' +v'dp +udn use this result to write an expression for the change in enthalpy of a two-phase (alpha + beta) system. if the entropy, pressure, and total number of moles are constrained to be constant, then the criterion for equilibrium is that the enthalpy is a minimum. paraphrase the strategy used to deduce the conditions for equilibrium in an isolated system to derive them for a system constrained to constant s', p and n. what happens to the condition for mechanical equilibrium?

Answers: 2


Another question on Physics

Physics, 21.06.2019 22:50
Moon effect. some people believe that the moon controls their activities. if the moon moves from being directly on the opposite side of earth from you to being directly overhead, by what percentage does (a) the moon's gravitational pull on you increase and (b) your weight (as measured on a scale) decrease? assume that the earth–moon (center-to-center) distance is 3.82 × 10^8 m, earth's radius is 6.37 × 10^6 m, moon's mass is 7.36 × 10^22 kg, and earth's mass is 5.98 × 10^24 kg.
Answers: 2

Physics, 21.06.2019 23:00
How many dots must be added to the symbol to properly represent a standard nitrogen ion? a) 1 b) 3 c) 5 d) 8
Answers: 1


Physics, 22.06.2019 21:30
Which are causes of mechanical weathering? (check all that apply) a. acid rain b. plant growth c. animal actions d. carbon dioxide e. pressure release i chose b & e and got the question wrong.
Answers: 2
You know the right answer?
The combined statement of the first and second laws for the change in enthalpy of unary single-phase...
Questions






World Languages, 03.11.2020 05:50



History, 03.11.2020 05:50

Mathematics, 03.11.2020 05:50

History, 03.11.2020 05:50


Spanish, 03.11.2020 05:50




Mathematics, 03.11.2020 05:50


Mathematics, 03.11.2020 05:50

Mathematics, 03.11.2020 05:50

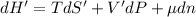
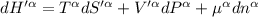
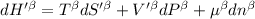
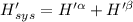
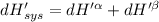
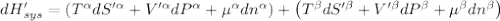
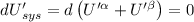
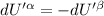
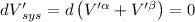
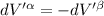
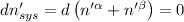
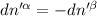
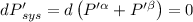
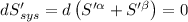
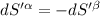
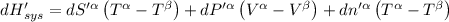
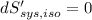
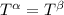 ----- for thermal equilibrium
----- for thermal equilibrium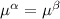 ----- for chemical equilibrium
----- for chemical equilibrium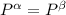 ----- for mechanical equilibrium
----- for mechanical equilibrium 

