
Physics, 02.12.2019 20:31 lavorisjonesjr1
Consider that 168.0 j of work is done on a system and 305.6 j of heat is extracted from the system. in the sense of the first law of thermodynamics, what is the value (including algebraic sign) of w, the work done by the system?

Answers: 1


Another question on Physics

Physics, 21.06.2019 21:40
Achemistry student needs of 40ml diethylamine for an experiment. by consulting the crc handbook of chemistry and physics, the student discovers that the density of diethylamine is 0.7 . calculate the mass of diethylamine the student should weigh out. round your answer to significant digits.
Answers: 1

Physics, 22.06.2019 05:30
If gases like carbon dioxide and methane make up less than 1% of the total atmosphere, why is it important for scientists to monitor changes in percentages of these gases?
Answers: 1

Physics, 22.06.2019 06:30
Air initially at 0.75 bar, 1000 k, and occupying a volume of 0.12 m^3 undergoes two processes. process 1-2: the air is compressed isothermally until the volume is halved. process 2-3: the air undergoes a constant pressure process until the volume is halved again. assume ideal gas behavior. a) determine the mass of the air, in kg. b) the work and the heat transfer for each of the two processes, in kj. (100 kj = 1 bar . m^3)
Answers: 1

Physics, 22.06.2019 08:00
Which graph represents motion with an object with positive velocity that is located at a position of 3 meters at a time of 0 seconds? a) a b) bb eliminate c) c d) d
Answers: 1
You know the right answer?
Consider that 168.0 j of work is done on a system and 305.6 j of heat is extracted from the system....
Questions


English, 11.11.2020 18:30


Social Studies, 11.11.2020 18:30

Mathematics, 11.11.2020 18:30

Spanish, 11.11.2020 18:30

Physics, 11.11.2020 18:30

Mathematics, 11.11.2020 18:30


Arts, 11.11.2020 18:30


Mathematics, 11.11.2020 18:30


English, 11.11.2020 18:30

History, 11.11.2020 18:30


Social Studies, 11.11.2020 18:30

Social Studies, 11.11.2020 18:30

Social Studies, 11.11.2020 18:30

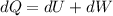
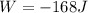 Work is done ON the system
Work is done ON the system 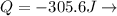 Heat is extracted FROM the system
Heat is extracted FROM the system

