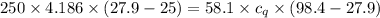Physics, 29.11.2019 06:31 ajbrock1004
A58.1g sample of quartz is put into a calorimeter that contains 250.0g of water. the quartz sample starts off at 98.4°c and the temperature of the water starts off at 25.0°c. when the temperature of the water stops changing it's 27.9°c. the pressure remains constant at 1 atm. calculate the specific heat capacity of quartz according to this experiment. be sure your answer is rounded to the correct number of significant digits.

Answers: 1


Another question on Physics

Physics, 21.06.2019 21:30
One day, after pulling down your window shade, you notice that sunlight is passing through a pinhole in the shade and making a small patch of light on the far wall. you see that the patch of light seems to be a circular diffraction pattern. it appears that the central maximum is about 1 cm across and you estimate that the distance from the window shade to the wall is about 3 m. what is (a) the average wavelength of sunlight? (b) the diameter of the pinhole?
Answers: 3

Physics, 22.06.2019 03:00
The respiratory system removes oxygen and water from the body. select the best answer from the choices provided t f
Answers: 2

Physics, 22.06.2019 11:30
If forces acting on an object are unbalanced. true or false
Answers: 1

You know the right answer?
A58.1g sample of quartz is put into a calorimeter that contains 250.0g of water. the quartz sample s...
Questions

Chemistry, 29.01.2020 12:45

History, 29.01.2020 12:45


Mathematics, 29.01.2020 12:45

Mathematics, 29.01.2020 12:45

Computers and Technology, 29.01.2020 12:45





English, 29.01.2020 12:45

History, 29.01.2020 12:45


Physics, 29.01.2020 12:45

Social Studies, 29.01.2020 12:45


Mathematics, 29.01.2020 12:45



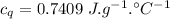
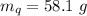 mass of water in calorie-meter,
mass of water in calorie-meter, 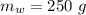 initial temperature of quartz,
initial temperature of quartz, 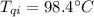 initial temperature of water,
initial temperature of water, 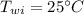 final temperature of the mixture,
final temperature of the mixture, 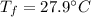
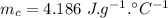
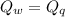
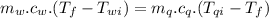
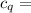 specific heat capacity of quartz
specific heat capacity of quartz