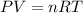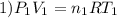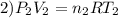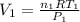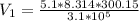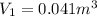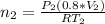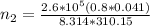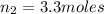Physics, 28.11.2019 01:31 nonispn606
Imagine a car tire that contains 5.1 moles of air when at a gauge pressure of 2.1×10^5n/m2 (the pressure above atmospheric pressure) and a temperature of 27 degrees c. the temperature increases to 37 degrees c, the volume decreases to 0.8 times the original volume, and the gauge pressure decreases to 1.6×10^5n/m2.
how many moles of air are left in the tire?

Answers: 2


Another question on Physics

Physics, 21.06.2019 20:00
What happens to atoms and chemical bonds during a reaction?
Answers: 1

Physics, 22.06.2019 12:00
Aboat radioed a distress call to a coast guard station. at the time of the call, a vector a from the station to the boat had a magnitude of 45.0 km and was directed 15.0° east of north. a vector from the station to the point where the boat was later found is = 30.0 km, 15.0° north of east. what are the components of the vector from the point where the distress call was made to point where the boat was found? in other words, what are the components of vector c = b - a?
Answers: 3

Physics, 22.06.2019 16:30
Each neuron shown in this figure innervates a group of muscle fibers. what is the term for a group of muscle fibers innervated by a single neuron?
Answers: 1

Physics, 22.06.2019 20:00
Bahan yang digunakan mencegah terjadinya polarisasi pada batu baterai a.larutan h2so4b.mncl2 dan serbuk karbonc.pbso4d.nh4cl
Answers: 3
You know the right answer?
Imagine a car tire that contains 5.1 moles of air when at a gauge pressure of 2.1×10^5n/m2 (the pres...
Questions

Mathematics, 27.09.2021 14:00

Mathematics, 27.09.2021 14:00

Mathematics, 27.09.2021 14:00

Mathematics, 27.09.2021 14:00


English, 27.09.2021 14:00





Computers and Technology, 27.09.2021 14:00


English, 27.09.2021 14:00



Mathematics, 27.09.2021 14:00

Social Studies, 27.09.2021 14:00

Mathematics, 27.09.2021 14:00


Mathematics, 27.09.2021 14:00