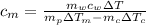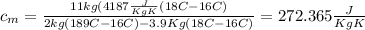Calculate the specific heat of a metal from the following data. a container made of the metal has a mass of 3.9 kg and contains 11 kg of water. a 2.0 kg piece of the metal initially at a temperature of 189°c is dropped into the water. the container and water initially have a temperature of 16.0°c, and the final temperature of the entire system is 18.0°c.

Answers: 3


Another question on Physics

Physics, 22.06.2019 02:00
What is created when solids,liquids,an gases mix with one another
Answers: 1

Physics, 22.06.2019 05:30
An object weighs 40n in air, weighs 20n when submerged in water and 30n when submerged in a liquid of unknown density. what is the density of the liquid?
Answers: 2

Physics, 22.06.2019 18:00
Consider an ideal gas at 27.0 degrees celsius and 1.00 atmosphere pressure. imagine the molecules to be uniformly spaced, with each molecule at the center of a small cube. what is the length l of an edge of each small cube if adjacent cubes touch but don't overlap?
Answers: 2

Physics, 22.06.2019 18:00
Astudent pushes a 60-n block across the floor for a distance of 10 m. how much work was done to move the block
Answers: 1
You know the right answer?
Calculate the specific heat of a metal from the following data. a container made of the metal has a...
Questions


History, 05.07.2019 21:50

Geography, 05.07.2019 21:50

German, 05.07.2019 21:50

History, 05.07.2019 21:50



Chemistry, 05.07.2019 21:50


Mathematics, 05.07.2019 21:50



History, 05.07.2019 22:00


Mathematics, 05.07.2019 22:00

Mathematics, 05.07.2019 22:00


Mathematics, 05.07.2019 22:00

Mathematics, 05.07.2019 22:00

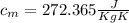
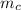 = mass of the container = 3.9 kg
= mass of the container = 3.9 kg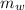 = mass of the water inside of the container= 11 kg
= mass of the water inside of the container= 11 kg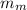 =mass of the metal= 2 kg
=mass of the metal= 2 kg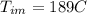 initital temperature of the metal
initital temperature of the metal initital temperature of the water
initital temperature of the water initital temperature of the container
initital temperature of the container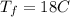 final equilibrium temperature
final equilibrium temperature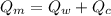
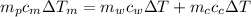
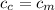
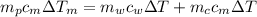
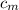 from the last expression we got:
from the last expression we got: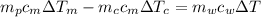
![c_m [m_p \Delta T_m -m_c \Delta T_c] =m_w c_w \Delta T](/tpl/images/0387/5562/73942.png)