
In the laboratory a "coffee cup" calorimeter, or constant pressure calorimeter, is frequently used to determine the specific heat of a solid, or to measure the energy of a solution phase reaction. a student heats 69.12 grams of tungsten to 98.93 °c and then drops it into a cup containing 85.45 grams of water at 23.82 °c. she measures the final temperature to be 25.63 °c. the heat capacity of the calorimeter (sometimes referred to as the calorimeter constant) was determined in a separate experiment to be 1.56 j/°c. assuming that no heat is lost to the surroundings calculate the specific heat of tungsten.

Answers: 1


Another question on Physics

Physics, 20.06.2019 18:04
Which career professionals are part of the architecture and construction career cluster? check all that apply. a. civil engineers b. carpenters c. landscapers d. plumbers e. surveyors f. civil drafters
Answers: 1

Physics, 22.06.2019 08:00
What is a carrier wave and how does it affect what you hear on the radio
Answers: 1

Physics, 22.06.2019 09:30
Astone is dropped from a cliff and falls 9.44 meters. what is the speed of the stone when it reaches the ground? a. 13.6 m/sec b. 1.39 m/sec c. 185 m/sec d. 9.80 m/sec
Answers: 3

Physics, 22.06.2019 12:00
The sun’s mass is 2.0×10^ 30 kg, its radius is 7.0×10 5 km, and it has a rotational period of approximately 28 days. if the sun should collapse into a white dwarf of radius 3.5×10 3 km, what would its period be if no mass were ejected and a sphere of uniform density can model the sun both before and after?
Answers: 3
You know the right answer?
In the laboratory a "coffee cup" calorimeter, or constant pressure calorimeter, is frequently used t...
Questions



Mathematics, 01.04.2021 05:50

Mathematics, 01.04.2021 05:50


Mathematics, 01.04.2021 05:50

Physics, 01.04.2021 05:50

History, 01.04.2021 05:50

Mathematics, 01.04.2021 05:50

Physics, 01.04.2021 05:50






Computers and Technology, 01.04.2021 05:50

Mathematics, 01.04.2021 06:00



Mathematics, 01.04.2021 06:00

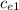 = 128.3 J / kg ° C
= 128.3 J / kg ° C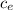 ΔT
ΔT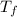 ) = (m₂
) = (m₂ 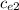 + C) (
+ C) (

