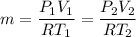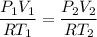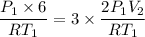Physics, 22.11.2019 05:31 yesharabaskoro
Asample of an ideal gas initially occupies a volume of 6 l. the pressure of the sample is then doubled while it is cooled to one third its original absolute temperature. after these changes, what volume does the sample occupy?

Answers: 3


Another question on Physics

Physics, 21.06.2019 23:20
Imagine you had to physically add electrons, one at a time, to a previously neutral conductor. you add one electron very easily, but the second electron requires more work. in your initial post to the discussion, explain why this is. also, what happens to the work needed to add the third, fourth, fifth, and subsequent electrons
Answers: 1

Physics, 22.06.2019 03:30
Will give brainliest! jay rides his 2.0-kg skateboard. he is moving at speed 5.8 m/s when he pushes off the board and continues to move forward in the air at 5.4 m/s. the board now goes forward at 13 m/s.a. determine jay’s mass.b. determine the change in the internal energy of the system during this process.(express your answer to two significant figures and include the appropriate units.)
Answers: 1

Physics, 22.06.2019 09:40
Aturntable a is built into a stage for use in a theatrical production. it is observed during a rehearsal that a trunk b starts to slide on the turntable 10 s after the turntable begins to rotate. knowing that the trunk undergoes a constant tangential acceleration of 0.31 m/s2, determine the coefficient of static friction between the trunk and the turntable.
Answers: 3

You know the right answer?
Asample of an ideal gas initially occupies a volume of 6 l. the pressure of the sample is then doubl...
Questions



Mathematics, 18.09.2021 19:20

Mathematics, 18.09.2021 19:20

Computers and Technology, 18.09.2021 19:20

Mathematics, 18.09.2021 19:20

Geography, 18.09.2021 19:20

Mathematics, 18.09.2021 19:20




Social Studies, 18.09.2021 19:20


Mathematics, 18.09.2021 19:20

Mathematics, 18.09.2021 19:20

Social Studies, 18.09.2021 19:20

Mathematics, 18.09.2021 19:20

Mathematics, 18.09.2021 19:20

Mathematics, 18.09.2021 19:20