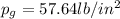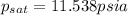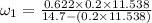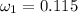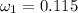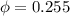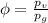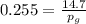Physics, 19.11.2019 06:31 fqjenfiq6959
Amixture of nitrogen and water vapor at 200of, 1 atm has the molar analysis of 80% n2, 20% water vapor. a) if the mixture is cooled at constant pressure, determine the temperature, in of, at which water vapor begins to condense. b) if the mixture is compressed at constant temperature, determine the pressure, in atm, at which water vapor begins to condense.

Answers: 3


Another question on Physics



Physics, 22.06.2019 16:00
The electric field direction is defined by the direction of the force felt by (select one of the following answers): 1. a negative charge. 2. a positive charge. 3. both positive and negative charges.
Answers: 2

You know the right answer?
Amixture of nitrogen and water vapor at 200of, 1 atm has the molar analysis of 80% n2, 20% water vap...
Questions







English, 20.05.2021 06:30



Mathematics, 20.05.2021 06:30


Mathematics, 20.05.2021 06:30





Mathematics, 20.05.2021 06:30

Mathematics, 20.05.2021 06:30