
Physics, 14.11.2019 23:31 melaniegilbreath
The reaction mixture represented above is at equilibrium at 298 k. the value of the equilibrium constant for the reaction is 16 at 298 k. if r(g), x(g) and z(g) each have an equilibrium concentration of 2.0 m, what is the equilibrium concentration of t(g)?

Answers: 1


Another question on Physics

Physics, 20.06.2019 18:02
5points ! is potential chemical energy always transformed into kinetic
Answers: 1

Physics, 22.06.2019 03:00
Lymphocytes known as blastocysts make antibodies that fight infection. select the best answer from the choices provided t f
Answers: 2

Physics, 22.06.2019 09:30
Asap i'm in class rn a 1,000-kg car is traveling 20 m/s on a flat stretch of road. it gets to a hill and coasts uphill until it stops. how high up the hill does the car travel? givens: equation: 1/2mv2initial=mghfinal solve for h. plug & chug, label.
Answers: 2

Physics, 22.06.2019 11:30
(1 point) match the differential equations and their vector valued function solutions. you may wish to multiply at least one solution out fully, to make sure that you know how to do it. you can get the other answers quickly by process of elimination and just multiply out one row element.
Answers: 2
You know the right answer?
The reaction mixture represented above is at equilibrium at 298 k. the value of the equilibrium cons...
Questions

French, 30.09.2019 14:30

Business, 30.09.2019 14:30

Biology, 30.09.2019 14:30

History, 30.09.2019 14:30

History, 30.09.2019 14:30

History, 30.09.2019 14:30

Mathematics, 30.09.2019 14:30


History, 30.09.2019 14:30

English, 30.09.2019 14:30


Mathematics, 30.09.2019 14:30

Social Studies, 30.09.2019 14:30

History, 30.09.2019 14:30

Social Studies, 30.09.2019 14:30

History, 30.09.2019 14:30



History, 30.09.2019 14:30

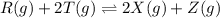
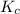 will be,
will be,![K_c=\frac{[Z][X]^2}{[R][T]^2}](/tpl/images/0374/7917/68fe3.png)
![16=\frac{(2.0)\times (2.0)^2}{(2.0)\times [T]^2}](/tpl/images/0374/7917/78a91.png)
![[T]=0.5M](/tpl/images/0374/7917/1fb66.png)


