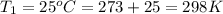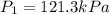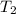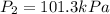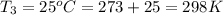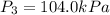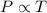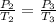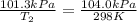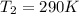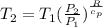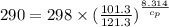Physics, 12.11.2019 02:31 jackiecroce1
Calculate the heat capacity of a gas sample from the following information: the sam- ple comes to equilibrium in a flask at 25°c and 121.3 kpa. a stopcock is opened briefly, allowing the pressure to drop to 101.3 kpa. with the stopcock closed, the flask warms, returning to 25°c, and the pressure is measured as 104.0 kpa. determine cp in j·mol−1·k−1 assuming the gas to be ideal and the expansion of the gas remaining in the flask to be reversible and adiabatic.

Answers: 3


Another question on Physics

Physics, 21.06.2019 22:30
Which expression allows you to determine the mechanical advantage of an inclined plane? a. height of plane / input force b. length of plane / input force c. length of plane / height of plane d. height of plane / length of plane
Answers: 1

Physics, 22.06.2019 07:30
30 points - when can a theory be modified if a new type of technology allows for new observations that raise new questions? a. immediately, while the questions about the theory are being asked b. after new hypotheses related to the theory are tested in experiments c. only after the new observations disprove all parts of the theory d. while scientists begin to think about how the theory could improve
Answers: 1


Physics, 22.06.2019 17:00
Amajor difference radio waves, visible light, and gamma rays is the of the photons, which results in different photon frequencies and wavelengths
Answers: 1
You know the right answer?
Calculate the heat capacity of a gas sample from the following information: the sam- ple comes to e...
Questions

Arts, 03.12.2020 03:20

English, 03.12.2020 03:20

Mathematics, 03.12.2020 03:20


Mathematics, 03.12.2020 03:20



Mathematics, 03.12.2020 03:20

History, 03.12.2020 03:20

Mathematics, 03.12.2020 03:20

Mathematics, 03.12.2020 03:20

Mathematics, 03.12.2020 03:20



History, 03.12.2020 03:20

Arts, 03.12.2020 03:20



History, 03.12.2020 03:20

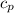 for reversible and adiabatic expansion is 55.04 J/mol.K
for reversible and adiabatic expansion is 55.04 J/mol.K