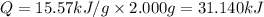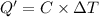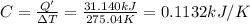Physics, 11.11.2019 20:31 clickbaitdxl
Constant-volume calorimeters are sometimes calibrated by running a combustion reaction of known δe and measuring the change in temperature. for example, the combustion energy of glucose is 15.57 kj/g. when a 2.000 g sample of glucose burns in a constant volume calorimeter, the calorimeter temperature increases from 21.45 to 23.34°c. find the total heat capacity of the calorimeter (in kj/k).

Answers: 1


Another question on Physics

Physics, 22.06.2019 08:30
A40.0 l tank of ammonia has a pressure of 12.7 kpa. calculate the. volume of the ammonia if it’s pressure is changed to 8.4 kpa while its temperature remains constant.
Answers: 3

Physics, 22.06.2019 11:10
Which situation will produce the greatest change of momentum
Answers: 2

Physics, 22.06.2019 12:10
Consider a one meter long horizontal pipe with a constant 100 cm^2 cross sectional area. water flows rightward into the pipe at x = 0 with flow velocity 02m/sec at every point within the pipe intake area. at x=1, the rightward flow rate is 0.192 m/sec. assume the water is a conserved quantity in the pipe, so there must be a leak (a sink) somewhere in the pipe. 1. compute net volumetric flow of the source if the system to be in equilibrium. 2. now assume the pipe in the problem has no leaks. compute the net volumetric rate of change for the system.
Answers: 3

Physics, 22.06.2019 12:30
What is the power rating of the lightbulb if 3.0 a flow through it when connected to a 15 v battery
Answers: 1
You know the right answer?
Constant-volume calorimeters are sometimes calibrated by running a combustion reaction of known δe a...
Questions



Mathematics, 26.03.2020 15:58

Medicine, 26.03.2020 15:58


Mathematics, 26.03.2020 15:58


Mathematics, 26.03.2020 15:58

Mathematics, 26.03.2020 15:58

Social Studies, 26.03.2020 15:58


History, 26.03.2020 15:58

Mathematics, 26.03.2020 15:58

Mathematics, 26.03.2020 15:58

Mathematics, 26.03.2020 15:58



Engineering, 26.03.2020 15:59

History, 26.03.2020 15:59

Mathematics, 26.03.2020 15:59