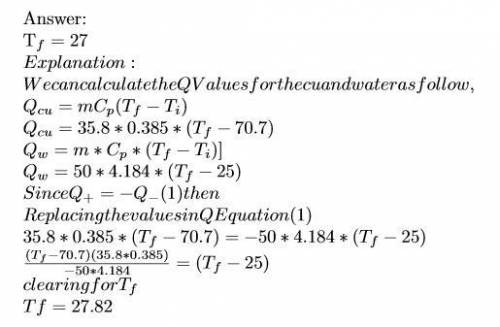
Ahot lump of 35.8 g of copper at an initial temperature of 70.7 °c is placed in 50.0 ml h2o initially at 25.0 °c and allowed to reach thermal equilibrium. what is the final temperature of the copper and water, given that the specific heat of copper is 0.385 j/(g·°c)? assume no heat is lost to surroundings.

Answers: 2


Another question on Physics

Physics, 22.06.2019 09:00
The material that keeps its new shape after it is stretched is called?
Answers: 1

Physics, 22.06.2019 12:20
A2.0 μf capacitor and a 4.0 μf capacitor are connected in series across a 0.827-kv potential. the charged capacitors are then disconnected from the source and connected to each other with terminals of like sign together. find the voltage across each capacitor.
Answers: 2

Physics, 22.06.2019 20:40
Newtons view of arrangement of the universe. don't copy from anywhere.
Answers: 3

Physics, 22.06.2019 22:30
You add 800 ml of water at 20c to 800 ml of water at 80c what is the most likely final temperature of the mixture ? a. 100c b. 60c c. 25 c d.50c
Answers: 1
You know the right answer?
Ahot lump of 35.8 g of copper at an initial temperature of 70.7 °c is placed in 50.0 ml h2o initiall...
Questions

Mathematics, 04.09.2019 18:30

English, 04.09.2019 18:30





Chemistry, 04.09.2019 18:30




Social Studies, 04.09.2019 18:30


English, 04.09.2019 18:30




Spanish, 04.09.2019 18:30

Social Studies, 04.09.2019 18:30

Mathematics, 04.09.2019 18:30