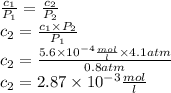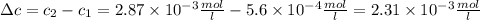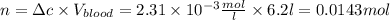The solubility of n2 in blood at 37°c and a partial pressure of 0.80 atm is 5.6 ✕ 10−4 mol·l−1. a deep-sea diver breathes compressed air with a partial pressure of n2 equal to 4.1 atm. assume that the total volume of blood in this diver's body is 6.2 l. calculate the amount of n2 gas released (in liters) when the diver returns to the surface of water, where the partial pressure of n2 is 0.80 atm. (2 sig fig)

Answers: 3


Another question on Physics



Physics, 22.06.2019 12:30
Brades exam guidelines exam instructions billing & payments & support question 6 of 20 : select the best answer for the question use this illustration to answer the question below. forms & resources al programs 9 ww linics 6. in the circuit shown in the figure above, suppose that the value of r, is 100k, and the value of r2 is 470 kq. at which of the following locations in the circuit would you measure the highest voltage with your meter? rvices 999999 a. between points b and c b. between points a and b c. between points b and e d. between points a and c eredu mark for review (will be highlighted on the review page) mend < < previous question next question
Answers: 1

Physics, 22.06.2019 15:10
Suppose that f : rn → rm and that a ∈ k, where k is a connected subset of rn . suppose further that for each x ∈ k there exists a δx > 0 such that f(x) = f(y) for all y ∈ bδx (x). prove that f is constant on k; that is, f(x) = f(a) for all x ∈ k
Answers: 1
You know the right answer?
The solubility of n2 in blood at 37°c and a partial pressure of 0.80 atm is 5.6 ✕ 10−4 mol·l−1. a de...
Questions

Mathematics, 01.09.2020 20:01



Social Studies, 01.09.2020 20:01

Social Studies, 01.09.2020 20:01




Mathematics, 01.09.2020 20:01

Social Studies, 01.09.2020 20:01




Biology, 01.09.2020 20:01


Biology, 01.09.2020 20:01

Social Studies, 01.09.2020 20:01

Mathematics, 01.09.2020 20:01

Physics, 01.09.2020 20:01