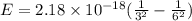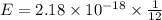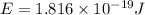An electron in a hydrogen atom undergoes a transition from the n = 3 level to the n = 6 level. to accomplish this, energy, in the form of light, must be absorbed by the hydrogen atom. calculate the energy of the light (in kj/photon) associated with this transition.

Answers: 1


Another question on Physics

Physics, 22.06.2019 04:10
Atotal charge of –6.50 µc is uniformly distributed within a sphere that has a radius of 0.150 m. what is the magnitude and direction of the electric field at 0.300 m from the surface of the sphere? a) 2.89 × 105 n/c, radially inward b) 6.49 × 105 n/c, radially outward c) 4.69 × 105 n/c, radially inward d) 9.38 × 105 n/c, radially outward e) 1.30 × 106 n/c, radially inward
Answers: 3

Physics, 22.06.2019 18:30
Ahot-air balloon is 11.0 m above the ground and rising at a speed of 7.00 m/s. a ball is thrown horizontally from the balloon basket at a speed of 9.00 m/s. ignore friction and air resistance and find the speed of the ball when it strikes the ground.
Answers: 1

Physics, 23.06.2019 07:00
What is the period of a wave traveling 5 m/s if its wavelength is 20 m/s
Answers: 2

Physics, 23.06.2019 11:10
If an object is thrown in an upward direction from the top of a building 1.6 x 102 ft. high at an initial velocity of 21.82 mi/h, what is its final velocity when it hits the ground?
Answers: 2
You know the right answer?
An electron in a hydrogen atom undergoes a transition from the n = 3 level to the n = 6 level. to ac...
Questions


Computers and Technology, 30.05.2021 22:30

Mathematics, 30.05.2021 22:30


English, 30.05.2021 22:30



French, 30.05.2021 22:30

Mathematics, 30.05.2021 22:30



Mathematics, 30.05.2021 22:30

Mathematics, 30.05.2021 22:30

Mathematics, 30.05.2021 22:30

English, 30.05.2021 22:30





Mathematics, 30.05.2021 22:30