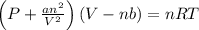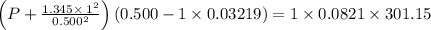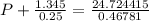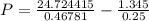Physics, 30.10.2019 00:31 miguel454545
If 1.00 mol of argon is placed in a 0.500-l container at 28.0 ∘c , what is the difference between the ideal pressure (as predicted by the ideal gas law) and the real pressure (as predicted by the van der waals equation)? for argon, a=1.345(l2⋅atm)/mol2 and b=0.03219l/mol.

Answers: 1


Another question on Physics

Physics, 22.06.2019 18:20
An object dropped from cliff falls with a constant acceleration of 10 metre per second square find its speed of two seconds after it was dropped
Answers: 2

Physics, 22.06.2019 18:40
Which body is in equilibrium? (1) a satellite orbiting earth in a circular orbit (2) a ball falling freely toward the surface of earth (3) a car moving with a constant speed along a straight, level road (4) a projectile at the highest point in its trajectory
Answers: 2

Physics, 22.06.2019 22:00
Acar is traveling 35 mph on a smooth surface . if a balanced force is applied to the car what happens?
Answers: 3

You know the right answer?
If 1.00 mol of argon is placed in a 0.500-l container at 28.0 ∘c , what is the difference between th...
Questions

Mathematics, 17.03.2021 23:50



Computers and Technology, 17.03.2021 23:50




Computers and Technology, 17.03.2021 23:50

Mathematics, 17.03.2021 23:50



Chemistry, 17.03.2021 23:50

Social Studies, 17.03.2021 23:50




Mathematics, 17.03.2021 23:50

Chemistry, 17.03.2021 23:50

Chemistry, 17.03.2021 23:50

History, 17.03.2021 23:50