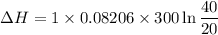One mole of a gas is placed in a closed system with a 20 l vessel initially at t = 300 k. the vessel is then isothermally expanded to 40 l. the gas follows the equation of state: p = rt/v + a/v2 where a = 240 l2 · atm/mol2 and r = 0.08206 l · atm/ mol · k. a. derive an expression relating (dh/dv)t to measurable properties. b. find dh for the gas in this process.

Answers: 2


Another question on Physics

Physics, 22.06.2019 03:10
Aphysical change is a change in the size, shape,, or stafe of matter true or false
Answers: 1

Physics, 22.06.2019 06:30
The energy of a photon was found to be 3.38 × 10–19 j. planck’s constant is 6.63 × 10–34 j • s. which color of light corresponds to this photon?
Answers: 2

Physics, 22.06.2019 22:00
On mars a rock falls an unknown vertical distance from a resting position and lands in a crater. if it takes the rock 2.5 seconds to fall, how high is the cliff the rock fell from? mars' surface gravity is 3.8 m/s2.
Answers: 2

Physics, 23.06.2019 03:20
When a pendulum is held high and taut and then is released, the pendulum begins to swing. what’s the correct order of the energy transformations in this example? (pe stands for potential energy.)
Answers: 2
You know the right answer?
One mole of a gas is placed in a closed system with a 20 l vessel initially at t = 300 k. the vessel...
Questions

Mathematics, 13.07.2019 05:00

Mathematics, 13.07.2019 05:00


History, 13.07.2019 05:00

Mathematics, 13.07.2019 05:00




Mathematics, 13.07.2019 05:00

Mathematics, 13.07.2019 05:00

Mathematics, 13.07.2019 05:00

English, 13.07.2019 05:00

Mathematics, 13.07.2019 05:00

Mathematics, 13.07.2019 05:00



Mathematics, 13.07.2019 05:00

Geography, 13.07.2019 05:00

Geography, 13.07.2019 05:00

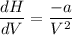
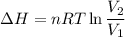 (ΔU=0)
(ΔU=0)