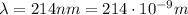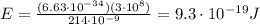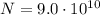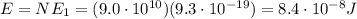Physics, 29.10.2019 19:31 cindykulei3719
The emission line used for zinc determinations in atomic emission spectroscopy is 214 nm. if there are 9.00×1010 atoms of zinc emitting light in the instrument flame per second, what energy (in joules) must the flame supply during this time to achieve this level of emission?

Answers: 2


Another question on Physics

Physics, 22.06.2019 09:00
The pressure proportional to the area a- inversely b- directly c- increase d-decrease
Answers: 2

Physics, 22.06.2019 09:00
What is a possible result of higher air temperature caused by global warming
Answers: 1

Physics, 22.06.2019 18:30
Anonzero net force acts on a particle and does work. which one of the following statements is true? the kinetic energy of the particle changes, but the speed of the particle does not change. the kinetic energy of the particle does not change, but the speed of the particle does change. the kinetic energy of the particle changes, but the velocity of the particle does not change. the kinetic energy and the speed of the particle change, but the velocity of the particle does not change. the kinetic energy, speed, and velocity of the particle change.
Answers: 1

Physics, 23.06.2019 01:30
The electric field inside a cell membrane is 8.0mn/c. what's the force on a singly charged ion in this field?
Answers: 1
You know the right answer?
The emission line used for zinc determinations in atomic emission spectroscopy is 214 nm. if there a...
Questions


Mathematics, 02.08.2019 00:10



Mathematics, 02.08.2019 00:10



Mathematics, 02.08.2019 00:10



Geography, 02.08.2019 00:10

Geography, 02.08.2019 00:10



Mathematics, 02.08.2019 00:10

Mathematics, 02.08.2019 00:10




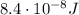
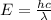
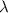 is the wavelength of the photon
is the wavelength of the photon