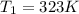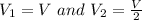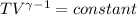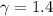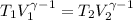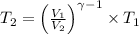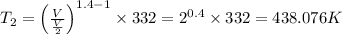Avertical cylinder is divided into two parts by a movable piston of mass m. the piston and cylinder system is well insulated (that is, no heat can flow in or out of the system) and the piston is initially held at rest. the top part of the cylinder is evacuated and the bottom part is filled with 1.00 mole of diatomic ideal gas at temperature 332 k. after the piston is released and the system comes to equilibrium, the volume occupied by gas is halved. find the final temperature of the gas.

Answers: 3


Another question on Physics

Physics, 22.06.2019 10:00
Aria drove to the store, did some shopping, and then came home. during maria's trip, when was her displacement equal to zero?
Answers: 1

Physics, 22.06.2019 20:20
An electron is trapped at a defect in a crystal. the defect may be modeled as a one-dimensional, rigid-walled box of width 1.00 nm. (a) sketch the wavefunctions and probability densities for the n 1 and n 2 states. (b) for the n 1 state, nd the probability of nding the electron between x1 0.15 nm and x2 0.35 nm, where x 0 is the left side of the box. (c) repeat (b) for the n 2 state. (d) calculate the energies in electron volts of the n 1 and n 2 states
Answers: 1

Physics, 22.06.2019 23:00
The gun from the crime scene shows tool marks where the serial numbers are ground off. there are also tool marks on the bullets left behind. what information can the tool marks on the bullets and gun provide about the crime? what type of evidence does this information provide, individual or class?
Answers: 1

Physics, 23.06.2019 11:30
In a house all the electrical wires run back to either a bank or a blank
Answers: 1
You know the right answer?
Avertical cylinder is divided into two parts by a movable piston of mass m. the piston and cylinder...
Questions



English, 30.03.2020 03:30

Mathematics, 30.03.2020 03:30



Mathematics, 30.03.2020 03:30

Mathematics, 30.03.2020 03:30

English, 30.03.2020 03:30



Social Studies, 30.03.2020 03:30

Mathematics, 30.03.2020 03:30


Business, 30.03.2020 03:30

English, 30.03.2020 03:31

Mathematics, 30.03.2020 03:31

Chemistry, 30.03.2020 03:31