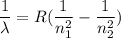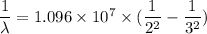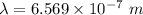The energy of the electron in a hydrogen atom can be calculated from the bohr formula: in this equation stands for the rydberg energy, and stands for the principal quantum number of the orbital that holds the electron. (you can find the value of the rydberg energy using the data button on the aleks toolbar.) calculate the wavelength of the line in the absorption line spectrum of hydrogen caused by the transition of the electron from an orbital with to an orbital with . round your answer to significant digits.

Answers: 3


Another question on Physics

Physics, 21.06.2019 18:10
Keneila is attempting to ski down a 20 m high friction free hill for the first time. she has a speed 10 m/s at the top. what is her kinetic energy when she is a the bottom, 20 m ? (a)2500 j (b)9800 j (c)12300 j (d)3100j (e)15000j
Answers: 2


Physics, 22.06.2019 10:00
The rocket is fired vertically and tracked by the radar station shown. when θ reaches 66°, other corresponding measurements give the values r = 32700 ft, r¨ = 85 ft/sec2, and θ˙ = 0.019 rad/sec. calculate the magnitudes of the velocity and acceleration of the rocket at this position.
Answers: 3

You know the right answer?
The energy of the electron in a hydrogen atom can be calculated from the bohr formula: in this equa...
Questions


Geography, 18.03.2021 01:30

English, 18.03.2021 01:30


Chemistry, 18.03.2021 01:30

History, 18.03.2021 01:30

English, 18.03.2021 01:30




History, 18.03.2021 01:30

English, 18.03.2021 01:30


Mathematics, 18.03.2021 01:30

Chemistry, 18.03.2021 01:30

Mathematics, 18.03.2021 01:30

Mathematics, 18.03.2021 01:30

Mathematics, 18.03.2021 01:30

Mathematics, 18.03.2021 01:30

Arts, 18.03.2021 01:30

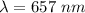
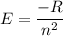 .............(1)
.............(1)