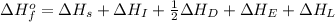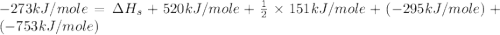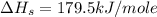Physics, 07.10.2019 18:10 dorafacegirl
Consider the following: li(s) (g) n lii(s) 222 kj. lii(s) has a lattice energy of kj/mol. the ionization energy of li(g) is 520. kj/mol, the bond en- ergy of i2(g) is 151 kj/mol, and the electron affinity of i(g) is kj/mol. use these data to determine the heat of sublimation of li(s

Answers: 1


Another question on Physics


Physics, 22.06.2019 09:40
(a) assume the equation x = at^3 + bt describes the motion of a particular object, with x having the dimension of length and t having the dimension of time. determine the dimensions of the constants a and b. (use the following as necessary: l and t, where l is the unit of length and t is the unit of time.) (b) determine the dimensions of the derivative dx/dt = 3at^2 + b. (use the following as necessary: l and t, where l is the unit of length and t is the unit of time.)
Answers: 1

Physics, 22.06.2019 15:30
Ineed ! using proper grammar, spelling, and punctuation, write at least one 5 sentence paragraph describing 3 ways we use the elements of the electromagnetic spectrum (ems) in our everyday lives.
Answers: 1

Physics, 22.06.2019 22:00
Ultraviolet radiation is dangerous because it has a high enough energy to damage skin cells. what is the best explanation of the relationship between wavelength and wave energy? a) wave energy is related only to frequency, not wavelength. b) longer wavelengths have lower frequencies but higher energy. c) the shorter the wavelength, the lower the frequency and the higher the energy. d) the shorter the wavelength, the higher the frequency and the higher the energy.
Answers: 3
You know the right answer?
Consider the following: li(s) (g) n lii(s) 222 kj. lii(s) has a lattice energy of kj/mol. the...
Questions

Mathematics, 06.07.2019 07:00

Chemistry, 06.07.2019 07:00


Social Studies, 06.07.2019 07:00


Chemistry, 06.07.2019 07:00

Mathematics, 06.07.2019 07:00

History, 06.07.2019 07:00



English, 06.07.2019 07:00

English, 06.07.2019 07:00



Mathematics, 06.07.2019 07:00


Biology, 06.07.2019 07:00



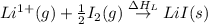
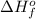 = enthalpy of formation of lithium iodide
= enthalpy of formation of lithium iodide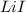 :
: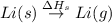
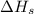 = sublimation energy of lithium
= sublimation energy of lithium 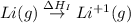
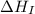 = ionization energy of lithium
= ionization energy of lithium 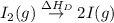
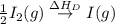
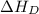 = dissociation energy of iodine
= dissociation energy of iodine 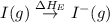
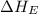 = electron affinity energy of iodine
= electron affinity energy of iodine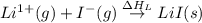
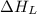 = lattice energy of lithium iodide
= lattice energy of lithium iodide