
Acoffee-cup calorimeter is used to determine the heat of reaction for the reaction of compound a with compound b. a(aq) + b(aq) → c(aq) when we add 16.10 ml of 0.189 m a at 23.722°c to 16.10 ml of 0.189 m b already in the calorimeter at the same temperature, the resulting temperature is observed to be 33.637°c. the heat capacity of the calorimeter has previously been determined to be 30.7 j/°c. assume that the specific heat of the mixture is the same as that of water, 4.184 j/g·°c, and that the density of the mixture is 1.00 g/ml. how much heat, in joules, was released by the reaction? (here we're looking for the magnitude of the heat. your answer should be positive.)

Answers: 3


Another question on Physics

Physics, 22.06.2019 02:30
Herbivores, carnivores, and omnivores are all types a. decomposers b. producers c. consumers d. biomes
Answers: 2

Physics, 22.06.2019 10:00
Explain the comparisons between nature and packingtown that appears on pages 81 and 82 answer
Answers: 2

Physics, 22.06.2019 11:30
Considering only the earth's rotation, determine how much later the asteroid would have had to arrive to put the explosion above helsinki at longitude 25˚ e? this would have obliterated the city.
Answers: 1

Physics, 22.06.2019 15:30
The radius of a sphere is increasing at a rate of 9 cm/ sec. find the radius of the sphere when the volume and the radius of the sphere are increasing at the same numerical rate.
Answers: 1
You know the right answer?
Acoffee-cup calorimeter is used to determine the heat of reaction for the reaction of compound a wit...
Questions

English, 23.04.2020 20:17

Arts, 23.04.2020 20:17



Mathematics, 23.04.2020 20:17

Social Studies, 23.04.2020 20:17


History, 23.04.2020 20:17


Physics, 23.04.2020 20:17

Social Studies, 23.04.2020 20:17


Mathematics, 23.04.2020 20:17


Mathematics, 23.04.2020 20:17





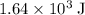 .
.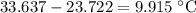 .
.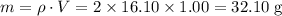 .
.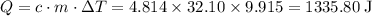 .
.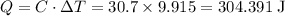 .
.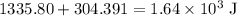 .
.

