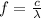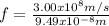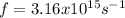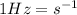Our understanding of the h atom will us learn about atoms with more electrons. the n =1 electron energy level of a h atom has an energy of 2.18 10–18 j. (a) what is the energy of the n = 5 level? (b) calculate the wavelength and frequency of a photon emitted when an electron jumps down from n = 5 to n = 1 in a h atom.

Answers: 1


Another question on Physics

Physics, 22.06.2019 04:50
Unpolarized light whose intensity is 1.19 w/ is incident on a polarizer. (a) what is the intensity of the light leaving the polarizer? (b) if the analyzer is set at an angle of = 41.0∘ with respect to the polarizer, what is the intensity of the light that reaches the photocell?
Answers: 1

Physics, 22.06.2019 05:30
Suppose you have three polarizing filters, with the second at an angle of 42∘ to the first and the third at an angle of 90∘ to the first. by what perfect will the original intensity of unpolarized light be reduced to after passing through all three filters?
Answers: 2


Physics, 23.06.2019 01:30
Lacie kicks a football from ground level at a velocity of 13.9 m/s and at an angle of 25.0° to the ground. how long will the ball be in the air before it lands? round your answer to the nearest tenth. s how far will the football travel before it lands? round your answer to the nearest tenth. m
Answers: 3
You know the right answer?
Our understanding of the h atom will us learn about atoms with more electrons. the n =1 electron en...
Questions


Mathematics, 13.10.2020 06:01



Mathematics, 13.10.2020 06:01


Mathematics, 13.10.2020 06:01




History, 13.10.2020 06:01


Mathematics, 13.10.2020 06:01



SAT, 13.10.2020 06:01

Chemistry, 13.10.2020 06:01

Mathematics, 13.10.2020 06:01

Spanish, 13.10.2020 06:01

Geography, 13.10.2020 06:01

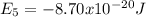
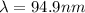 ,
, 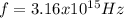
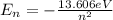 (1)
(1)
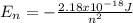
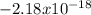 represents the energy of the ground state¹ and n is the principal quantum number.
represents the energy of the ground state¹ and n is the principal quantum number.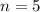 :
: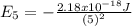
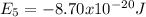
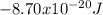 .
. 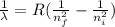 (2)
(2)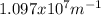
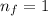 and
and 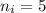 :
: 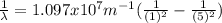
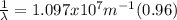
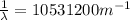
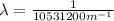
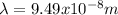
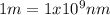
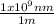
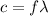 (3)
(3)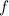 :
: