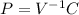When dealing with 1 liter of argon as a closed system and as an ideal gas that undergoes an isothermal expansion, which of the following expressions can be used to describe the state of your thermodynamic system? group of answer choices a. pv = r. b. pv = const. c. p is inverse proportional to v. d. answers (a) and (b) are both correct and they give the same information about the system under investigation. e. answers (b) and (c) are both correct and they give the same information about the system under investigation.

Answers: 3


Another question on Physics

Physics, 22.06.2019 03:00
The passage that directs air to the lungs is called a. diaphragm. b. larynx. c. bronchi. d. pharynx.
Answers: 2

Physics, 22.06.2019 09:00
An open cart is moving along a straight frictionless horizontal track. when rain starts falling vertically into the cart, what happens to the speed of the cart?
Answers: 3

Physics, 22.06.2019 14:40
Asolid cylinder and a cylindrical shell have the same mass, same radius, and turn on frictionless, horizontal axles. the cylindrical shell has light-weight spokes connecting the shell to the axle. a rope is wrapped around each cylinder and tied to blocks of equal masses that are held the same height above the ground. both blocks are released simultaneously. the ropes do not slip. which block hits the ground first? or is it a tie?
Answers: 3

Physics, 22.06.2019 20:50
In a game of pool, ball a is moving with a velocity v0 of magnitude v0 = 15 ft/s when it strikes balls b and c, which are at rest and aligned as shown. knowing that after the collision the three balls move in the directions indicated and assuming frictionless surfaces and perfectly elastic impact (that is, conservation of energy), determine the magnitudes of the velocities va, vb, and vc.
Answers: 3
You know the right answer?
When dealing with 1 liter of argon as a closed system and as an ideal gas that undergoes an isotherm...
Questions

Social Studies, 10.07.2019 04:00

History, 10.07.2019 04:00

History, 10.07.2019 04:00







Mathematics, 10.07.2019 04:00


Chemistry, 10.07.2019 04:00

Chemistry, 10.07.2019 04:00



Biology, 10.07.2019 04:00

Business, 10.07.2019 04:00