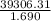Physics, 20.09.2019 23:20 laqu33n021
Chemical engineer studying the properties of fuels placed 1.690 g of a hydrocarbon in the bomb of a calorimeter and filled it with o2 gas. the bomb was immersed in 2.550 l of water and the reaction initiated. the water temperature rose from 20.00°c to 23.55°c. if the calorimeter (excluding the water) had a heat capacity of 403 j/k, what was the heat of reaction for combustion (qv) per gram of the fuel? (d for water = 1.00 g/ml; c for water = 4.184 j/g·°c.) enter your answer in scientific notation.

Answers: 2


Another question on Physics

Physics, 21.06.2019 14:10
Aman pushes on a trunk with a force of 250 newtons. the trunk does not move. how much positive work is done on the trunk? 0.0 j -250 j 250 j 125 j
Answers: 1

Physics, 22.06.2019 19:30
Coal contains energy. a. light b. kinetic c. chemical d. mechanical
Answers: 1

Physics, 23.06.2019 13:30
A6.5-g iron meteor hits the earth at a speed of 295 m/s. if its kinetic energy is entirely converted to heat in the meteor, by how much will its temperature rise? the specific heat of iron is 113 cal/kg ∙ c°, and 1 cal = 4.186 j. a) 57,100 c° b)384 c° c)0.147 c° d)92.0 c°
Answers: 1

Physics, 23.06.2019 14:00
What is the magnitude of the resultant vector? round your answer to the nearest tenth. 5 m 13 m
Answers: 3
You know the right answer?
Chemical engineer studying the properties of fuels placed 1.690 g of a hydrocarbon in the bomb of a...
Questions





Arts, 11.02.2020 08:49






Mathematics, 11.02.2020 08:49


World Languages, 11.02.2020 08:50


Mathematics, 11.02.2020 08:50

English, 11.02.2020 08:50

Mathematics, 11.02.2020 08:51

Chemistry, 11.02.2020 08:51

Mathematics, 11.02.2020 08:51