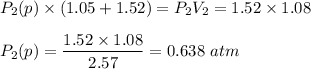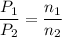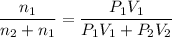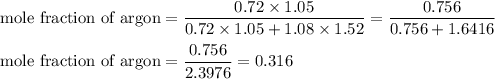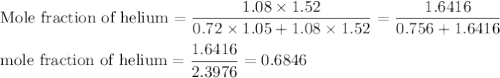A1.05-l bulb and a 1.52-l bulb are connected by a stopcock and filled, respectively, with argon at 0.72 atm and helium at 1.08 atm at the same temperature. calculate the total pressure, the partial pressures of each gas, and the mole fraction of each gas after the stopcock has been opened. assume ideal-gas behavior. (answer in 3 significant figures)

Answers: 3


Another question on Physics

Physics, 21.06.2019 22:30
Acricket ball of 70g moving with a velocity of 0.5 m/s is stopped by a player in 0.5s what is the force applied to stop the ball
Answers: 1

Physics, 22.06.2019 05:30
What tightening torque should be used for a hexagonal head screw (not split-bolt) on a 250 kcmil conductor?
Answers: 3

Physics, 22.06.2019 17:00
If a negatively charged particle is placed at rest in an electric potential field that increases in the positive x-direction, what will the particle do? a. accelerate in the positive x-direction b. remain at rest c. accelerate in the negative x-direction
Answers: 3

You know the right answer?
A1.05-l bulb and a 1.52-l bulb are connected by a stopcock and filled, respectively, with argon at 0...
Questions

History, 24.03.2021 14:00

Biology, 24.03.2021 14:00

Computers and Technology, 24.03.2021 14:00

Social Studies, 24.03.2021 14:00



Mathematics, 24.03.2021 14:00




Biology, 24.03.2021 14:00

Mathematics, 24.03.2021 14:00

Mathematics, 24.03.2021 14:00


Business, 24.03.2021 14:00



Geography, 24.03.2021 14:00

Mathematics, 24.03.2021 14:00

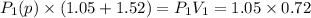
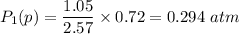
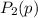 be the partial pressure of gas in bulb B then
be the partial pressure of gas in bulb B then