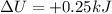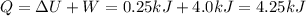Physics, 18.09.2019 03:20 tommyewall34
Agas contained within a piston-cylinder assembly, initially at a volume of 0.1 m3 , undergoes a constant-pressure expansion at 2 bars to a final volume of 0.12 m3 , while being slowly heated through the base. the change in internal change of the gas 0.25 kj. the piston and cylinder walls are fabricated from heat-resistant material and the piston moves smoothly in the cylinder. for the gas as the system, evaluate work and heat transfer each in kj (neglect the potential energy change and kinetic energy change).

Answers: 2


Another question on Physics

Physics, 22.06.2019 07:00
Critical mass is the of material required to produce a chain reaction. a.) minimum amount b.) atomic mass c.) precise amount d.) maximum amount
Answers: 1

Physics, 22.06.2019 10:00
In a heat engine if 1000 j of heat enters the system the piston does 500 j of work, what is the final internal energy of the system if the initial energy was 2000 j? 1. write the equation 2.list out your known variables 3.plug the numbers into the equations 4.solve 5.write your solution statement that includes initial energy and final
Answers: 3

Physics, 22.06.2019 15:20
Aphoton is absorbed by an electron that is in the n = 3 state of a hydrogen atom, causing the hydrogen atom to become ionized. very far away from the nucleus, the released electron has a velocity of 750,000 m/s. what was the wavelength of the absorbed photon?
Answers: 2

Physics, 22.06.2019 18:30
Adolphin emits ultrasound at 100khz and uses the timing of reflections to determine the position of objects in the water. part a what is the wavelength of this ultrasound? assume that temperature of water is 20 degrees c. answer in cm
Answers: 2
You know the right answer?
Agas contained within a piston-cylinder assembly, initially at a volume of 0.1 m3 , undergoes a cons...
Questions



Physics, 21.01.2020 17:31


Mathematics, 21.01.2020 17:31




History, 21.01.2020 17:31

Mathematics, 21.01.2020 17:31


English, 21.01.2020 17:31





Biology, 21.01.2020 17:31


Biology, 21.01.2020 17:31

Mathematics, 21.01.2020 17:31

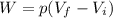
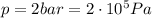 is the pressure
is the pressure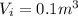 is the initial volume
is the initial volume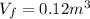 is the final volume
is the final volume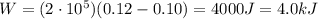
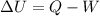
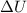 is the change in internal energy of the gas
is the change in internal energy of the gas