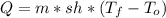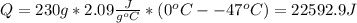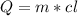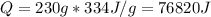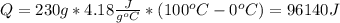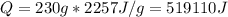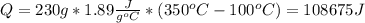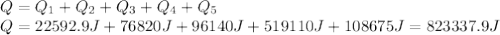Physics, 17.09.2019 23:20 7thaohstudent
Water has the following thermodynamic constants: (1) specific heat liquid = 4.18 j/g °c, solid = 2.09 j/g °c, gas = 1.89 j/g °c, (2) heat of fusion = 334 j/g, and (3) heat of vaporization = 2257 j/g. for a sample of water at 1.0 atm of pressure, mass = 230 g at an initial temperature of -47 °c and a final temperature of 350 °c, answer the following questions: (1) how much heat is required to warm the solid sample to its melting point? j (2) how much heat is required to melt the sample? j (3) how much heat is required to warm the liquid sample to its boiling point? j (4) how much heat is required to vaporize the sample? j (5) how much heat is required to warm the gaseous sample to its final temperature? j and finally, (6) how much heat is required for the entire process to occur

Answers: 2


Another question on Physics

Physics, 22.06.2019 16:30
Each neuron shown in this figure innervates a group of muscle fibers. what is the term for a group of muscle fibers innervated by a single neuron?
Answers: 1

Physics, 22.06.2019 19:30
Which type of energy would have nothing to do with ironing clothes? a. heat b. chemical c. electrical d. mechanical
Answers: 1

Physics, 22.06.2019 19:30
Acamcorder has a power rating of 20 watts. if the output voltage from its battery is 9 volts, what current does it use?
Answers: 2

Physics, 22.06.2019 19:30
Complete these sentences. if a roller coaster train has a potential energy of 1,500 j and a kinetic energy of 500 j as it starts to travel downhill, its total energy isj. once the roller coaster train gets closer to the bottom of the hill, its kinetic energy increases to 1,100 j, and its potential energy decreases to j. when the train reaches the bottom of the track and is traveling along the ground, its kinetic energy isj.
Answers: 1
You know the right answer?
Water has the following thermodynamic constants: (1) specific heat liquid = 4.18 j/g °c, solid = 2....
Questions

Biology, 21.07.2019 05:40





History, 21.07.2019 05:50


Mathematics, 21.07.2019 05:50


Social Studies, 21.07.2019 05:50




History, 21.07.2019 05:50

History, 21.07.2019 05:50



History, 21.07.2019 05:50

Mathematics, 21.07.2019 05:50