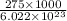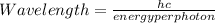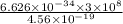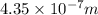Physics, 16.09.2019 20:30 nayiiii1874
The energy required to dislodge electrons from sodium metal via the photoelectric effect is 275 kj/mol . what wavelength of light, in nanometers, has sufficient energy per photon to dislodge an electron from the surface of sodium? express the wavelength in nanometers to three significant figures.

Answers: 1


Another question on Physics

Physics, 22.06.2019 00:00
Name three different units of energy used to measure heat and describe what type of situations each is usually used.
Answers: 2

Physics, 22.06.2019 09:30
The necleus of an atom is made up of what subatomic particles?
Answers: 1

Physics, 22.06.2019 19:30
Emagnitude of the electrical force acting between a +2.4 × 10–8 c charge and a +1.8 × 10–6 c charge that are separated by 0.008 m is n, rounded to the tenths place.
Answers: 3

Physics, 23.06.2019 02:00
How is an electron dot structure used to represent a covalent bond?
Answers: 2
You know the right answer?
The energy required to dislodge electrons from sodium metal via the photoelectric effect is 275 kj/m...
Questions


History, 22.06.2020 23:55



English, 22.06.2020 23:55


Mathematics, 22.06.2020 23:55


History, 22.06.2020 23:55


Mathematics, 22.06.2020 23:55

Mathematics, 22.06.2020 23:55


Mathematics, 22.06.2020 23:55

Mathematics, 22.06.2020 23:55

Mathematics, 22.06.2020 23:55


Mathematics, 22.06.2020 23:55