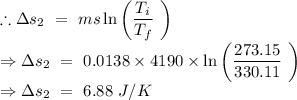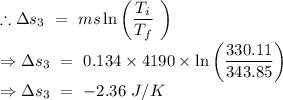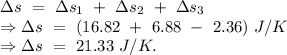Physics, 14.09.2019 02:30 kelseychristian24
An insulated thermos contains 134 g of water at 70.7°c. you put in a 13.8 g ice cube at 0.00°c to form a system of ice + original water. the specific heat of liquid water is 4190 j/kg*k; and the heat of fusion of water is 333 kj/kg. what is the net entropy change of the system from then until the system reaches the final (equilibrium) temperature?

Answers: 2


Another question on Physics

Physics, 21.06.2019 16:10
Amachine, modeled as a simple spring-mass system, oscillates in simple harmonic motion. its acceleration is measured to have an amplitude of 5,000 mm/s with a fre- quency of 10 hz. compute the maximum displacement the machine undergoes during this oscillation.
Answers: 1

Physics, 22.06.2019 05:00
Aperson walking 1 mile everyday for exercise, leaving her front porch at 9: 00 am and returning to her front porch at 9: 25 am. what is the total displacement of her daily walk? a) 1 mileb) 0c) 25 minutes d) none of the above
Answers: 1

Physics, 22.06.2019 09:30
The necleus of an atom is made up of what subatomic particles?
Answers: 1

Physics, 22.06.2019 11:30
(2) (a) you have a simple circuit that consists of only a battery (δvbat=1.5v) and two resistors with resistance r1=10ω and r2=5ω, connected in series with each other. what is the current running through the battery? (b) you re-arrange your circuit so now r2 is attached in parallel to r1 rather than in series. what is the current running through the battery? (c) you add an additional resistor r3=7ω on the same branch as r2. what is the current running through the battery? (d) what is the power dissipated in r3?
Answers: 3
You know the right answer?
An insulated thermos contains 134 g of water at 70.7°c. you put in a 13.8 g ice cube at 0.00°c to fo...
Questions

Health, 02.09.2019 12:10




French, 02.09.2019 12:10

Social Studies, 02.09.2019 12:10

Mathematics, 02.09.2019 12:10

Chemistry, 02.09.2019 12:10

Social Studies, 02.09.2019 12:10


Mathematics, 02.09.2019 12:10


Biology, 02.09.2019 12:10

Chemistry, 02.09.2019 12:10

Mathematics, 02.09.2019 12:10

Biology, 02.09.2019 12:10


Social Studies, 02.09.2019 12:10

Mathematics, 02.09.2019 12:10

History, 02.09.2019 12:10

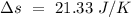
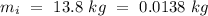 Temperature of the ice =
Temperature of the ice = 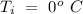 Mass of the original water =
Mass of the original water = 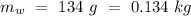 Temperature of the original water =
Temperature of the original water = 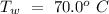 Specific heat of water =
Specific heat of water = 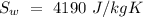 Latent heat of fusion of ice =
Latent heat of fusion of ice = 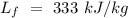
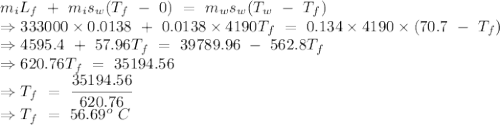
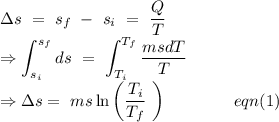
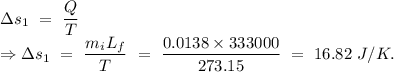
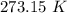 to water 330.11 K water.
to water 330.11 K water.