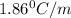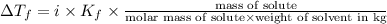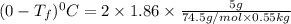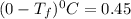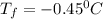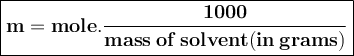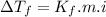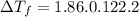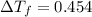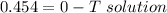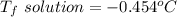Physics, 12.09.2019 19:30 Miloflippin9766
Part a calculate the freezing point of a solution containing 5.0 grams of kcl and 550.0 grams of water. the molal-freezing-point-depression constant (kf) for water is 1.86 °c/m. calculate the freezing point of a solution containing 5.0 grams of kcl and 550.0 grams of water. the molal-freezing-point-depression constant () for water is 1.86 °c/m. +0.23 °c -0.23 °c 1.23 °c -0.45 °c +0.45 °c

Answers: 3


Another question on Physics

Physics, 22.06.2019 00:30
Order the sequence of ideas that lead to marie curies discovery of radioactive elements number the events in chronological order starting with the oldest
Answers: 2

Physics, 22.06.2019 10:30
Astone weighing 1.5 kilograms is resting on a rock at a height of 20 meters above the ground. the stone rolls down 10 meters and comes to rest on a patch of moss. the gravitational potential energy of the stone on the moss is joules.
Answers: 1

Physics, 22.06.2019 14:30
Which compound is held together by the electrostatic force between two ions? a. co2 b. cci4 c. h2s d. mgf2
Answers: 1

Physics, 22.06.2019 18:20
Awave with a frequency of 500 hz is traveling at a speed of 100 m/s.what is the wavelength?
Answers: 1
You know the right answer?
Part a calculate the freezing point of a solution containing 5.0 grams of kcl and 550.0 grams of wat...
Questions

Mathematics, 12.10.2019 23:30


History, 12.10.2019 23:30

Mathematics, 12.10.2019 23:30

Mathematics, 12.10.2019 23:30

English, 12.10.2019 23:30

History, 12.10.2019 23:30

Mathematics, 12.10.2019 23:30


Physics, 12.10.2019 23:30

Engineering, 12.10.2019 23:30

Mathematics, 12.10.2019 23:30

Biology, 12.10.2019 23:30


Chemistry, 12.10.2019 23:30


English, 12.10.2019 23:30


Mathematics, 12.10.2019 23:30

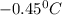
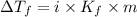
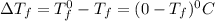 = Depression in freezing point
= Depression in freezing point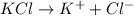
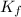 = freezing point constant =
= freezing point constant =